reactions with basic oxides are chemical phenomena in which a substance of this class of oxides is placed in the same container as a inorganic acid, a acid oxide or one amphoteric oxide.
See too:Reactions with amphoteric oxides
inorganic salt and water are common products in a reaction with basic oxides. To find out whether inorganic salt or water will be produced, we must look at the substance that accompanies the basic oxide in the reagent. See the examples below and understand how this process takes place.
Reactions with basic oxides and inorganic acids
In reactions between basic oxides and inorganic acids, an inorganic salt and water are formed, because the basic oxide cation (Y+) interacts with the anion (X-) of the acid, and the hydronium cation (H+) of the acid interacts with the anion of the oxide:
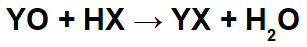
Example: Radium oxide and hydrobromic acid
In this reaction, we have radium oxide (RaO) and hydrobromic acid (Hbr) as reactants. Thus, the following interactions occur:
Radio cation (Ra+2) with the bromide anion (Br-1) forming the radium bromide salt (RaBr2).
Hydronium cation (H+1) with the oxide anion (O-2) forming water (H2O).
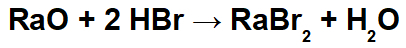
See too:Reactions with double oxides
Reactions with basic oxides and acid oxides
In these reactions, an inorganic salt is formed through the interaction between the basic oxide cation (Y+) and the resulting acid anion (WOThe-) of the chemical reaction between an acid oxide and water.
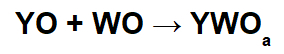
Example: Sodium oxide and sulfur dioxide
In this reaction, we have sodium oxide (Na2O) and sulfur dioxide (SO2). It is noteworthy that, initially, when the acid oxide interacts with water, we have the formation of sulfurous acid (H2ONLY3):

From the formation of this acid, the interaction between the sodium cation (Na+1) of the basic oxide and the sulfite anion (SO3-2) of the acid, forming the sodium sulphite salt (Na2ONLY3):
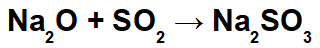
Reactions with basic oxides and amphoteric oxides
Amphoteric oxides are substances that exhibit dual chemical behavior: in the presence of an acidic substance, it behaves like a basic oxide; in the presence of a substance of a basic nature, it behaves like an acid oxide.
So when a basic oxide reacts with an amphoteric oxide, this will behave like an acid oxide, forming an inorganic acid in the presence of water. The reaction between the anion of the inorganic acid and the cation of the basic oxide will form a salt product.
Example: Potassium oxide and chromium III oxide
In this reaction, we have potassium oxide as reactants (K2O) and chromium oxide (Cr2O3). It is noteworthy that, initially, when amphoteric oxide interacts with water, we have the formation of chromosomal acid (2HCrO2):

From the formation of this acid, the interaction between the potassium cation occurs (K+1) of the basic oxide and the chromite anion (SO3-2) of the acid, forming the potassium chromite salt (2KCrO2):

By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-com-oxidos-basicos.htm

