Hydrocarbons are composed exclusively of carbon (C) and hydrogen (H) atoms, with a general formula: CxHy.
This is a frequent theme in university entrance exams and deserves attention in Chemistry studies.
Test your hydrocarbon knowledge with the following exercises. Also count on feedback and commented answers,
question 1
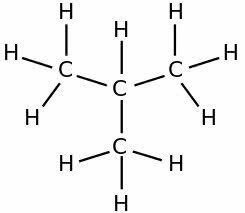
The correct name of the branched hydrocarbon, whose formula is outlined below is:
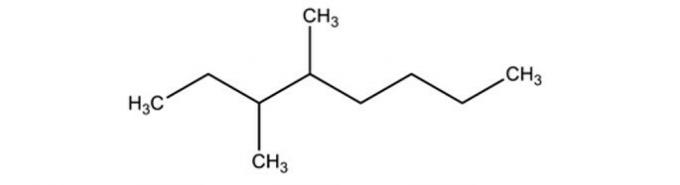
a) 3,4-diethyl-octene
b) 3,4-dimethyl-octane
c) 3,4-diethyl-octane
d) 3,4-dipropyl-octane
e) 3,4-dimethyl-octene
Correct alternative: b) 3,4-dimethyl-octane.
The first step in naming the hydrocarbon is to count the number of carbons that are present in the main chain.
To do this, we start counting from the end closest to the branches.
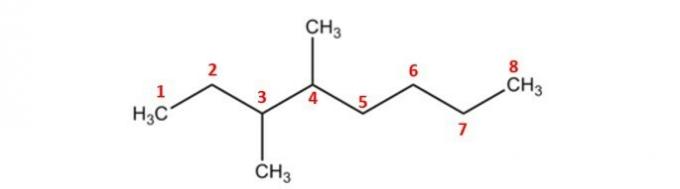
With carbons numbered from 1 to 8, identified on which carbons the methyl radicals (-CH) are present.3), which are carbons 3 and 4.
Therefore, the name of the compound is: 3,4-dimethyl-octane.
question 2
A medicinal plant used to regulate blood glucose is found in the Amazon region, popularly known as pata-de-vaca.
The species that works as a “vegetable insulin” has among its chemical compounds an alkane, whose formula contains 74 hydrogen atoms. Therefore, the number of carbon atoms present in the carbon chain is:
a) 33
b) 34
c) 35
d) 36
e) 37
Correct alternative: d) 36.
An alkane is a hydrocarbon formed by single bonds between its atoms. Since each carbon must make four bonds, the general formula for an alkane is CnoH2n + 2.
Knowing that the total number of hydrogens in the compound is 74, we find the value of n, which corresponds to the number of carbons.
2n + 2 = 74
2n = 74 - 2
2n = 72
n = 72/2
n = 36
So the number of carbons is 36. The compound mentioned in the question is n-hexatriacontane.
Read too: alkanes
question 3
Analyze the statements about hydrocarbons and respond with the sum of the correct alternatives.
(02) The boiling point of a series of hydrocarbons increases with increasing chain.
(04) Hydrocarbons are compounds formed by carbon and hydrogen atoms.
(16) Hydrocarbons are non-polar compounds and are therefore easily solubilized in water.
(32) The intermolecular interactions between hydrocarbon molecules are of the induced dipole type.
Correct answer: 02 + 04 + 32 = 38
02. CORRECT The boiling temperature of hydrocarbons increases with increasing molecular weight.
04. CORRECT The name itself indicates the junction of these two chemical elements: carbon and hydrogen. The carbon chain is formed by carbon atoms and hydrogens bond to them.
16. WRONG. Hydrocarbons are non-polar compounds and do not solubilize in water, a polar solvent, but in organic solvents.
32. CORRECT The induced dipole interaction makes the nonpolar molecules, with no difference in electronegativity, to stick together.
Read too: Hydrocarbons
question 4
(PUC-PR) Alkynes are hydrocarbons:
a) saturated aliphatics.
b) saturated alicyclics.
c) double bond unsaturated aliphatics.
d) triple bond unsaturated alicyclics.
e) triple-bonded unsaturated aliphatics.
Correct alternative: e) triple bond unsaturated aliphatics.
Aliphatic hydrocarbons are those that do not have a benzene ring in their chain. They can be cyclic when they have a ring in their structure. And also acyclic, when they do not have rings.
Furthermore, they can also be saturated and unsaturated. Saturated ones have only single bonds, and unsaturated ones have at least one unsaturation (double or triple bond).
Thus, alkynes are all acyclic and unsaturated hydrocarbons, which have a triple bond in their carbon chain.
Read too: Alkynes
question 5
(Unesp) Octane is one of the main constituents of gasoline, which is a mixture of hydrocarbons. The molecular formula of octane is:
a) C8H18
b) C8H16
c) C8H14
d) C12H24
e) C18H38
Correct alternative: a) C8H18
Remember that octane is an alkane, so it has only single bonds and an open chain. It has eight carbon and 18 hydrogen atoms in its formula.
Its structural formula is as follows:
question 6
(UFSCar) Consider the following statements about hydrocarbons.
I) Hydrocarbons are organic compounds consisting only of carbon and hydrogen.
II) Only straight-chain unsaturated hydrocarbons are called alkenes.
III) Cycloalkanes are saturated aliphatic hydrocarbons of general formula CnoH2n.
IV) Are aromatic hydrocarbons: bromobenzene, p-nitrotoluene and naphthalene.
The following statements are correct:
a) I and III, only.
b) I, III and IV only.
c) II and III only.
d) III and IV only.
e) I, II and IV only.
Correct alternative: a) I and III only.
The statement in item I is correct, as hydrocarbons are all compounds that have carbon and hydrogen in their constitution.
Item II is incorrect as alkynes are also unsaturated hydrocarbons. They have a triple bond in their carbon chain.
Item III is correct, as cycloalkanes or cyclans are hydrocarbons formed by single bonds and do not have an aromatic chain.
Item IV is incorrect, as bromobenzene and p-nitrotoluene are not hydrocarbons, they have other elements besides carbon and hydrogen.
question 7
(FATEC) Liquefied petroleum gas, LPG, is a mixture of propane, C3H8, and butane, C4H10. Therefore, this gas is a mixture of hydrocarbons of the class of:
a) alkanes.
b) alkenes.
c) alkynes.
d) cycloalkanes.
e) cycloalkenes.
Correct alternative: a) alkanes.
Propane and butane are alkanes, as they have only single bonds in their carbon chains and have a general formula CnoH2n + 2.
Propane:
Butane:
question 8
(Uel) The molecular formula of 2,3 - dimethyl butane is:
a) C6H14
b) C6H12
c) C6H10
d) C4H10
e) C4H8
Correct alternative: a) C6H14
Analyzing the name of the compound, we notice that it is formed by:
- But: 4 carbons
- An: single bonds between carbons
- 2,3 - dimethyl: two methyl radicals attached to carbons 2 and 3.
Therefore, the structural formula of 2,3 - dimethyl butane is:

Read too: Hydrocarbon Nomenclature
question 9
(UFU-MG) The substance of formula C8H16 represents a:
a) open-chain alkane.
b) open-chain alkene.
c) open-chain alkyne.
d) aromatic compound.
e) closed chain alkyne.
Correct alternative: b) open-chain alkene.
Observing the molecular formula of the compound, we can see that the number of hydrogens is twice the number of carbons, that is, CnoH2n.
Drawing the structural formula of the compound and remembering that carbon needs to make four bonds, we find that the compound is the octene.
The structural formula of octene, C8H16, é:

Read too: alkenes
question 10
(Unifor) 2-Methylpent-2-ene has a molecular formula:
a) C6H12.
b) C6H10.
c) C5H12.
d) C5H10.
e) C5H8.
Correct alternative: a) C6H12.
To determine the molecular formula let's first assemble the compound's chain.
The first step in answering this question is to distribute the carbons in the main chain. Pent-2-ene indicates that the main chain is formed by 5 carbons and "en" that there is a double bond at carbon 2.
Next, you must add the branch of the chain. 2 methyl indicates that a methyl radical (-CH) is also attached to carbon 23).
Knowing that carbon is tetravalent, complete the missing bonds in the compound by inserting hydrogen atoms.
The structural formula of 2-methylpent-2-ene is:
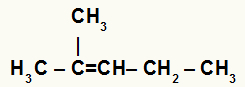
Therefore, when we count the atoms of the structure, we realize that the molecular formula of the compound is C6H12.
question 11
(Uema) OGX Energia, the Maranhão oil exploration arm of the EBX group, owned by businessman Eike Batista, discovered a giant natural gas reserve, a mixture of light hydrocarbons, consisting mainly of ethane, propane, isobutane, butane, pentane, isopentane, among others, in the city of Capinzal do Norte, located 260 km from St. Louis. The reserves, according to OGX, have 10 trillion to 15 trillion cubic feet of gas, the equivalent of 15 million cubic meters per day – half of what Bolivia sends to Brazil daily. Source: Available at: Accessed on: 01 Jul. 2013. (adapted)
The nomenclature of these light hydrocarbons, which constitute natural gas, is based, among some criteria, on the amount of carbon present in the compound. The correct number of carbons in the first six compounds mentioned in the text are, respectively:
a) 2, 5, 5, 3, 4, 4.
b) 2, 4, 4, 3, 5, 5.
c) 2, 4, 4, 5, 5, 3.
d) 2, 3, 5, 5, 4, 4.
e) 2, 3, 4, 4, 5, 5.
Correct alternative: e) 2, 3, 4, 4, 5, 5.
In order to know how many carbons exist in each of the compounds, we must know its structural or molecular formula.
Remember that the nomenclature of hydrocarbons helps to build their chains, as it indicates the number of carbons present and the presence or absence of branches and unsaturations.
In this case, we have:
Ethane: 2 carbons
Propane: 3 carbons

Isobutane: 4 carbons
Butane: 4 carbons

Pentane: 5 carbons

Isopentane: 5 carbons

Therefore, the correct alternative is: e) 2, 3, 4, 4, 5, 5.
question 12
(Uel) One of the formula C hydrocarbons5H12 may have carbon chain:
a) saturated cyclic.
b) heterogeneous acyclic.
c) branched cyclic.
d) unsaturated open.
e) open branched.
Correct alternative: e) open branched.
The hydrocarbon in question is an alkane, therefore, it has an open chain.
As the options suggest only unsaturated and branched open chain, and we know that alkanes do not have unsaturations. Therefore, it is possible to say that it is an open branched chain:
question 13
(Fatec) The label of a commercial solvent indicates that it contains only aliphatic hydrocarbons. From this information, it is concluded that this solvent should not contain, as one of its main components, the:
a) toluene.
b) n-hexane.
c) heptane.
d) cyclohexane.
e) pentane.
Correct alternative: a) toluene.
By the nomenclature of the compounds present in the alternatives, we can see that hexane, heptane, cyclohexane and pentane have the same -year termination.
This indicates that the compounds are formed by simple bonds between their carbons and, therefore, they are aliphatic hydrocarbons by the absence of aromatic structure, which corresponds to alternating double bonds in a cyclic chain, as occurs in the toluene.
Therefore, aliphatic hydrocarbons are those that have an open carbon chain. In this case, toluene is an aromatic hydrocarbon.
Read too: Aromatic hydrocarbons
question 14
(Fatec) The hydrocarbon that has the lowest amount of H atoms per molecule is:
a) methane.
b) ethane.
c) ethene.
d) etine.
e) bribe.
Correct alternative: d) etino.
The compounds ethane and propane are hydrocarbons of the alkane class. Therefore, they have the number of hydrogens greater than the number of carbons, as their general formula is CnoH2n+2.
Ethene has a double bond between the two carbons, as it is an alkene with the general formula CnoH2n.
Ethyne and propyne are alkyne, as they have a triple bond between carbons. However, propyne has a greater number of hydrogens, as it has one more carbon in the chain. The general formula for an alkyne is CnoH2n-2.
Therefore, ethyne has only two carbon atoms (C2H2), being the simplest alkyne.
question 15
(UEPB) Mothballs are products widely used in cabinets to fight moths. They decrease in size over time due to the phenomenon of sublimation. Check the alternative that corresponds to the chemical constituent of naphthalene and the organic series to which it belongs, respectively:
a) toluene; hydrocarbon.
b) naphthalene; cyclene.
c) phenanthrene; alkene.
d) naphthalene; aromatic hydrocarbon.
e) naphthol; phenol.
Correct alternative: d) naphthalene; aromatic hydrocarbon.
Naphthalene is one of the examples of an aromatic hydrocarbon.
Check out its structure below.
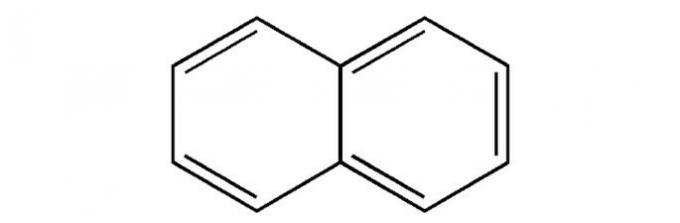
Read too:
- Organic chemistry
- Exercises on Organic Chemistry
- Exercises on Organic Functions

