Solubility is the physical property of substances to dissolve, or not, in a given liquid.
is called solute, chemical compounds that dissolve in another substance. O solvent it is the substance in which the solute will be dissolved to form a new product.
THE chemical dissolution is the process of dispersing the solute in a solvent, giving rise to a homogeneous solution or mixture.
Solutes can be classified into:
- Soluble: are the solutes that dissolve in the solvent.
- Slightly soluble: are the solutes that are difficult to dissolve in the solvent.
- Insoluble: are the solutes that do not dissolve in the solvent.
A common principle in solubility is: "like dissolve like”. This means that a polar solute tends to dissolve in a polar solvent. The same is true for non-polar substances.
See some examples:
- Hydrocarbons, compounds present in gasoline, are non-polar and have little solubility in water, which is polar.
- Alcohols, such as ethanol and methanol, are polar due to the presence of oxygen in the carbon chain and, therefore, are soluble in water.
- Salts have different solubility. They can be classified into: soluble salt and practically insoluble salt.
Solubility Coefficient
O solubility coefficient (Cs) determines the maximum capacity of the solute that dissolves in a given amount of solvent. This, depending on the temperature conditions.
In summary, the solubility coefficient is the amount of solute needed to saturate a standard amount of solvent at a given condition.
For example, consider the following situation:
In a glass of salted water (NaCl), initially, the salt disappears into the water.
However, if more salt is added, at some point it will start to accumulate in the bottom of the glass.
This is because the water, which is the solvent, has reached its solubility limit and the maximum amount of concentration. This is also called saturation point.
The solute that remains at the bottom of the container and does not dissolve is called the bottom body or precipitate.
In relation to saturation point, the solutions are classified into three types:
- unsaturated solution: when the amount of solute is less than Cs.
- saturated solution: when the amount of solute is exactly the same as Cs. It's the saturation limit.
- supersaturated solution: when the amount of solute is greater than Cs.
Solubility Product
As we have seen, solubility represents the amount of solute dissolved in a solution. O solubility product (Kps) is an equilibrium constant directly related to solubility.
Its calculation allows you to determine whether a solution is saturated, unsaturated or saturated with a precipitate. This calculation is related to the dissolution equilibrium and the concentration of ions in the solution.
This is because the product of solubility refers to the dissolution balance of ionic substances.
understand more about Solute and Solvent.
Solubility Curve
The chemical solubility capacity of a substance subjected to temperature change is not linear. The variation in solubility capacity, as a function of temperature, is known as the solubility curve.
Most solid substances have their solubility coefficient increased with increasing temperature. Thus, the solubility of each material occurs proportionally, depending on the temperature.
Each substance has its own solubility curve for a particular solvent.
The solubility variation is considered linear when not under the influence of temperature. To know the variation, it is necessary to look at the solubility curve.
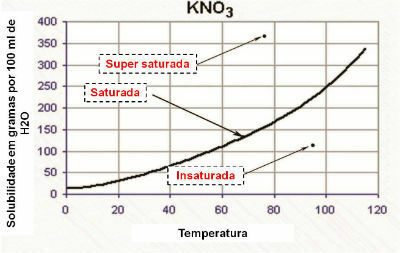
Solubility Curve
In the graph, the solubility curve demonstrates that the solution is:
- saturated: when the point is on the solubility curve.
- unsaturated: when the point is below the solubility curve.
- homogeneously saturated: when the point is above the solubility curve.
Also read about Solution Concentration.
Solubility Coefficient Formula
The formula to calculate the solubility coefficient is:
Cs = 100. m1/m2
Where:
Cs: solubility coefficient
m1: mass of solute
m2: solvent mass
Want to know more? read Chemical Solutions and Dilution of Solutions.
Exercises
1. (Fuvest-SP) A chemist read the following instruction in a procedure described in his laboratory guide:
"Dissolve 5.0 g of Chloride in 100 mL of water at room temperature..." .
Among the substances below, which one is mentioned in the text?
a) Cl2.
b) CCl4.
c) NaClO.
d) NH4Cl.
e) AgCl.
d) NH4Cl.
2. (UFRGS-RS) A certain salt has a solubility in water equal to 135g/L at 25°C. By completely dissolving 150 g of this salt in one liter of water, at 40°C, and slowly cooling the system to 25°C, a homogeneous system is obtained whose solution will be:
a) diluted.
b) concentrated.
c) unsaturated.
d) saturated.
e) supersaturated.
e) supersaturated.
3. (Mackenzie-SP) A typical example of a supersaturated solution is:
The mineral water.
b) homemade serum.
c) soda in a closed container.
d) alcohol 46° GL.
e) vinegar.
c) soda in a closed container.
4. (PUC-RJ) Note the figure below, which represents the solubility, in g per 100 g of H2O, of 3 inorganic salts in a given temperature range:
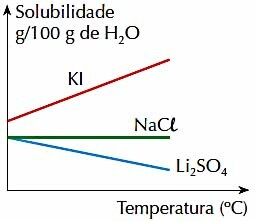
Check the correct statement:
a) The solubility of the 3 salts increases with temperature.
b) The increase in temperature favors the solubilization of Li2ONLY4.
c) The solubility of KI is greater than the solubilities of other salts, in the temperature range shown.
d) The solubility of NaCl varies with temperature.
e) The solubility of 2 salts decreases with temperature.
c) The solubility of KI is greater than the solubilities of other salts, in the temperature range shown.