Evaporation it is the passage from the liquid state to the gaseous state. It occurs on the free surface of liquids, slowly and gradually, at any temperature.
When a substance is in a liquid state, it has a lower cohesive force between its atoms than when it is in a solid state.
In this state, the molecules are further apart, in constant agitation and moving inside the liquid at different speeds.
In this way, the particles with greater speed, upon reaching the free surface of the liquid, manage to escape, passing to the gaseous state.

Evaporation Speed
There are factors that influence the speed at which evaporation occurs, they are:
- Temperature: the higher the temperature, the faster the evaporation speed. Because the higher the temperature, the higher the kinetic energy of the particles. In this way, more particles will escape from the liquid's surface.
- Nature of the liquid: there are substances that evaporate more easily, they are called volatile substances. Ether, alcohol and acetone are examples of volatile substances.
- free surface area: as evaporation takes place on the free surface of liquids, the larger the surface, the greater the amount of particles that will leave the liquid.
- Concentration of vapor on liquid: the greater the amount of steam, the lower the evaporation rate.
- Pressure exerted on the liquid: the higher the pressure, the lower the evaporation speed.
Difference between Evaporation and Boiling
Both evaporation and boiling represent the change from the liquid state to the gaseous state. However, while evaporation occurs gradually, boiling happens quickly.
For boiling to occur, the liquid must reach, for a given pressure, a certain temperature, called the boiling point. Evaporation can occur at any temperature.
Separation of Mixtures
Fractional crystallization is a process of separating heterogeneous mixtures. It is used when the substances that make up the mixture are in solid state.
In this process, a liquid that dissolves all the solid components is added to the mixture. The components then crystallize separately after the solution evaporates.
This process is used, for example, in salt pans to obtain salts from sea water.

Evaporation and the Water Cycle
Evaporation is one of the processes that constitute the water cycle. Energy from the Sun heats the free surface of lakes, rivers, seas and oceans.
This heating causes part of the water to evaporate into a vapor state. This, upon reaching the highest layers of the atmosphere, cools and condenses forming clouds.
When precipitation occurs, the water returns to the surface in liquid form, infiltrating the soil and forming underground sheets.
Part of this water is absorbed by plants, which return the water vapor to the atmosphere by transpiration.
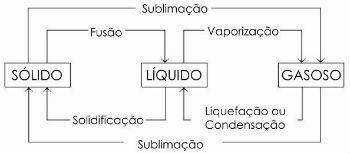
phase changes
The change from a liquid to a gaseous state is generically called vaporization, as it encompasses, in addition to evaporation, two other processes: boiling and heating.
There are still other processes of change of state. Are they:
- Fusion
- Solidification
- Liquefaction or Condensation
- Sublimation
In the diagram below we represent the three physical states of matter and the respective changes of state:
Learn more at: Physical State Changes.