The boiling temperature of organic compounds is influenced by two factors: the type of intermolecular interaction and the size of the molecule.
The larger the size of the molecule, the higher its boiling temperature.
Note the structures and their respective T.E.: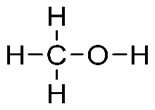 YOU. (°C): 64.5
YOU. (°C): 64.5
Methanol
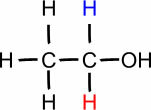 YOU. (°C): 78.3
YOU. (°C): 78.3
Ethanol
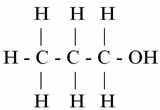 YOU. (°C): 97.2
YOU. (°C): 97.2
Propanol
Note that as the molecule increases the number of carbons, the boiling point becomes higher. Thus, methanol that has only one carbon atom boils at 64.5°C and Propanol (3 carbons) only reaches its T.E. at 97.2°C.
But in molecules of equal size? How to know which one has T.E. higher?
The type of intermolecular interaction will determine.
Example: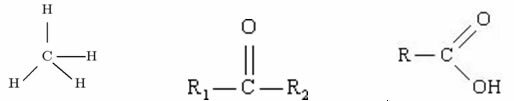
Methane Ketone Carboxylic Acid
Among these three organic structures, which one has the highest T.E.?
In this case, we cannot rely on the size of the molecule as we did before, as the three structures do not differ in this respect.
The rule that now applies is: The greater the intensity of the intermolecular forces, the higher the boiling temperature.
Induced-dipole < Dipole-dipole < Hydrogen bonds
Ascending order of intensity →
Based on this intensity scale, see which forces predominate in the molecules of:
Methane: dipole-induced
Ketone: dipole-dipole
Carboxylic acid: hydrogen bonds
With this we can classify which of these molecules has the highest boiling temperature: the Acid carboxylic acid has a higher T.E. because the hydrogen bond present in this compound has the greatest intensity.
Methane < Ketone < Carboxylic acid
Ascending order of intensity →
This scheme shows that Methane has the lowest boiling point due to the predominant type of intermolecular force (induced dipole).
By Líria Alves
Graduated in Chemistry
Brazil School Team
Do not stop now... There's more after the advertising ;)
See more!
Polarity of organic compounds
Organic chemistry - Chemistry - Brazil School
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Boiling temperature of organic compounds"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/temperatura-ebulicao-dos-compostos-organicos.htm. Accessed on June 28, 2021.

