Structural Formula is the scheme that indicates the structure, that is, the arrangement of the atoms that make up the chemical elements, as well as the connection between them. It can be represented in different ways: flat, condensed or electronic.
Among the factors that condition the structures used, the number of electrons in the valence shell is one of them.
Flat formula
The flat formula uses dashes to represent covalent bonds, which can be single, double or triple and represented in the following ways:
– simple connection (when 2 electrons are shared)
= double bond (when 4 electrons are shared)
≡ triple link (when 6 electrons are shared)
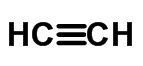
Condensed or Simplified Formula
In the condensed structural formula the bonds are not shown.
In its representation, the number of atoms of each element is indicated in a condensed way, that is, simplified:
H3C CH2 the CH2 CH3
Linear Condensed Formula
The linear condensed formula uses zigzag lines, at whose vertices the carbons are represented:
Electronic or Lewis Formula
The electronic formula, also called the Lewis formula, is represented by dots.
Through these points, the quantities of electrons present in the valence layers are shown:
H: H
And the Molecular Formula?
THE molecular formula, without referring to its structure, indicates the number of elements that make up a molecule. In addition to indicating the number of atoms present in each element and their proportions.
It can be obtained through the minimum or empirical formula and the percentage or centesimal formula.
Read too Isomerism and Valencia layer.
Solved Exercises
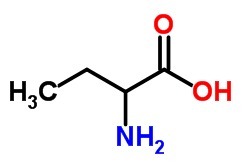
1. (Vunesp-2000) Write the structural formula and give the official name:
a) a saturated branched carbon chain ketone with a total of 7 carbon atoms.
b) an amino acid, with 4 carbon atoms.
The)

B)
2. (FGV-2005) Aspartame is an artificial sweetener that was accidentally discovered in 1965 by a careless chemist, who licked his dirty fingers and felt they were sweet.
These unhygienic habits are not recommended, as many substances in minute amounts are highly toxic.
The structural formula for aspartame is shown below:

From the structural formula of aspartame, it appears that there is
a) 13 carbon atoms per molecule.
b) 1 ether functional group.
c) 1 dipeptide
d) 2 tertiary carbon atoms
e) only 1 asymmetric carbon atom.
Alternative c: 1 dipeptide
To continue testing your knowledge, see also these exercise lists:
- Exercises on Hydrocarbons
- Exercises on Organic Chemistry
- Exercises on Flat Isomerism


