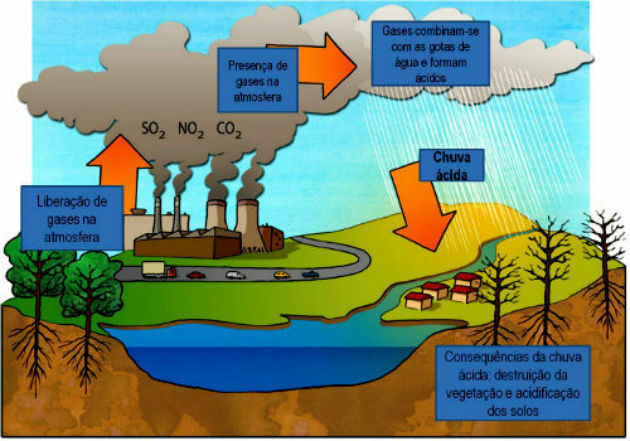
Acid rain is precipitation with the presence of sulfuric acid, nitric acid and nitrous, resulting from chemical reactions that occur in the atmosphere.
All rains are acidic, even in unpolluted environments. However, rainfall becomes an environmental problem when its pH is below 4.5.
They result from the exaggerated amount of products from burning fossil fuels released into the atmosphere as a result of human activities.
How is acid rain formed?
O carbon dioxide (CO2) existing in the atmosphere already makes rain slightly acidic, even in natural conditions. The natural pH of water is 7 and when in balance with CO2 atmospheric is 5.6, low acid.
the oxides of sulfur (ONLY2 and SO3) it's from nitrogen (N2O, NO and NO2) are the main components of acid rain. These compounds are released into the atmosphere through the burning of fossil fuels. When they react with water droplets from the atmosphere, they form sulfuric acid (H2ONLY4) and nitric acid (HNO3). Together, these two acids cause an increase in the acidity of rainwater.
See the chemical reactions that form these acids:
1. Formation of sulfuric acid:
2. Formation of nitric acid and nitrous acid:
In the presence of these acids, the pH of rainwater can reach between 4 and 2, extremely acidic values.
Learn more, read also:
- Air pollution
- What is Atmosphere?
Causes
Human activities are mainly responsible for this phenomenon of acid rain. As we have seen, the release of gases resulting from the use of fossil fuels is the main responsible for the formation of acid rain.
Thus, they are the result of the use of fossil fuels in transport, thermoelectric plants, industries and other forms of combustion. They can also be formed by natural causes, such as the release of gases during a volcano eruption.
Consequences
Industrialized countries are the most affected by acid rain. However, pollutants can be carried by air currents to distant locations.
This happened in Scandinavia's lakes, which were made acidic by the rains as a result of industrial activities in Germany, France and the United Kingdom.
For nature, the consequences of acid rain are the destruction of vegetation cover, acidification of soils and water in rivers and lakes.
An example of the consequence of acid rain was observed in Brazil. The coastal municipality of Cubatão, in São Paulo, has a large concentration of industries and acid rain has destroyed the vegetation on the slopes of the Serra do Mar, exposing the soil to erosion.
When acidification reaches the soil and waters of rivers and lakes, the living beings that inhabit these places are affected. Water and soil become unsuitable to house some organisms, leading to their death.
Acid rain can also corrode marble and limestone and oxidize metals in historic monuments such as buildings and statues.

Learn more about another environmental phenomenon caused by the excess of polluting gases in the atmosphere, the Greenhouse effect.

