Hydrocarbons are compounds formed only by carbon and hydrogen, with a general formula: CxHy.
It is an extensive amount of substances, the best known of which are constituents of oil and natural gas.
The backbone of a hydrocarbon is made up of carbon and, in turn, the hydrogen atoms bond through a covalent bond.
They are widely used in the chemical industry, being essential in the production of petroleum derivatives: fuels, polymers, paraffins, among others.
Properties of hydrocarbons
| molecular interaction | They are practically non-polar compounds and their molecules are joined by an induced dipole. |
|---|---|
| Melting and boiling point | They are low compared to polar compounds. |
| aggregation states |
|
| Density | They have a lower density than water. |
| Solubility | They are insoluble in water and soluble in non-polar substances. |
| Reactivity |
|
Classification of hydrocarbons
As for the form of the main carbon chain, hydrocarbons are classified into:
aliphatic hydrocarbons
Formed by open or acyclic carbon chains, which have terminal carbons.
- alkanes
- alkenes
- Alkynes
- Alkadienes
Example:
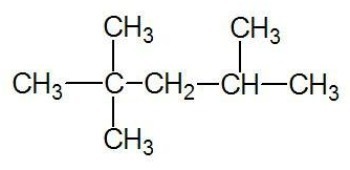
See too: carbon chains
Cyclic hydrocarbons
Formed by closed or cyclic carbon chains which do not have terminal carbons.
- Cyclans
- Cycles
- cyclists
- Aromatics
Examples:
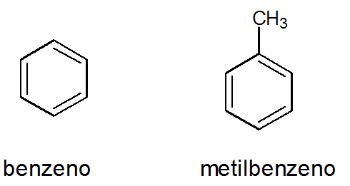
See too: benzene
As for the bonds of carbon chains, whether single, double or triple:
Saturated hydrocarbons
Compounds are formed by single bonds between carbon and hydrogen atoms.
- alkanes
- Cyclans
Example:
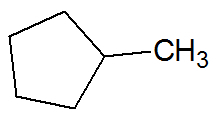
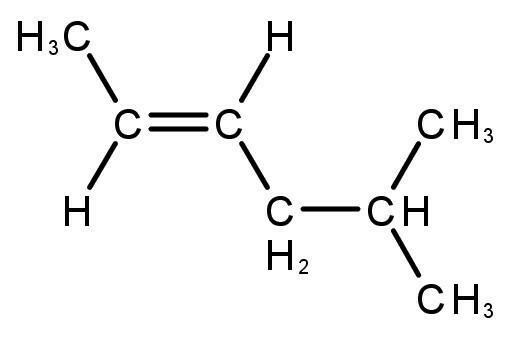
unsaturated hydrocarbons
The compounds formed have double or triple bonds between carbon and hydrogen atoms.
- alkenes
- Alkynes
- Alkadienes
- Cycles
- cyclists
- Aromatics
Also read about:
- Chemical bonds
- Organic chemistry
- Organic Functions
Nomenclature
The nomenclature of hydrocarbons is defined by the following terms:
| PREFIX | INTERMEDIARY | SUFFIX |
|---|---|---|
| Indicates the number of carbons present in the chain. | Type of link found in string. | Identification of the functional group. |
| PREFIX | INTERMEDIARY | SUFFIX | ||
|---|---|---|---|---|
| 1C | MET | Single link only | AN | O |
| 2C | ET | |||
| 3C | PROP | a double bond | EN | |
| 4C | BUT | |||
| 5C | PENT | two double bonds | DIEN | |
| 6C | HEX | |||
| 7C | HEPT | A triple bond | IN |
|
| 8C | OCT | |||
| 9C | NON | Two triple links | DIIN | |
| 10C | DEC |
Examples
Follow how hydrocarbon names are formed:
| Ethane |
- Prefix: ET, which corresponds to 2 carbons.
- Intermediary: AN, which corresponds to simple links.
- Suffix: O, which corresponds to the hydrocarbon function.
Look at the other examples:
| Propane | |
| Ethene | |
| Etino | |
| propadiene |
In some cases it is necessary to indicate the position of the double or triple bond. Numbering must start from the closest end of that link.
| 1,3-butadiene | |
| 1-butyne |
Learn more at:
- Butane
- Methane
Types of Hydrocarbons
Check out the main types of hydrocarbons, their characteristics and the compounds used:
alkanes
They are open chain hydrocarbons with single bonds between carbon and hydrogen atoms, whose general formula is ÇnoH2n+2.
Alkanes Characteristics
- They are also called paraffins or paraffins.
- They are found in nature in natural gas and oil.
- Are used as fuels: cooking gas, gasoline, diesel oil, etc.
Examples of alkanes

alkenes
They are open-chain hydrocarbons and have a double bond, whose general formula is ÇnoH2n.
Alkenes characteristics
- They are also called olefins, alkenes or ethylenic hydrocarbons.
- They are industrially obtained from the cracking of alkanes present in petroleum.
- They are used as raw material in industry: plastics, dyes, explosives, etc.
Examples of alkenes

Alkynes
They are open-chain hydrocarbons with the presence of a double bond, whose general formula is ÇnoH2n-2.
Alkynes Characteristics
- They are more reactive than alkanes and alkenes due to the triple bond.
- Alkynes with more than 14 carbon atoms are solid.
- The most used alkyne is acetylene, commonly used in the production of synthetic rubbers, textile fibers and plastics.
Examples of alkynes

Alkadienes
They are open-chain hydrocarbons and the presence of two double bonds, whose general formula is ÇnoH2n-2
Characteristics of alkadienes
- Also called dienes or diolefins
- They are found in nature in terpenes, which are extracted from essential fruit oils.
- The best known compound is isoprene, found in natural rubber and essential oils.
Examples of alkadienes

Cyclans
They are closed-chain hydrocarbons with single bonds between carbon and hydrogen atoms, whose general formula is ÇnoH2n.
Cyclan Characteristics
- They are also called cycloalkanes or cycloparaffins.
- They are unstable when subjected to high pressure.
- Chains with more than 6 carbons are stable, whereas chains with less than 5 carbons are reactive.
Examples of cyclans

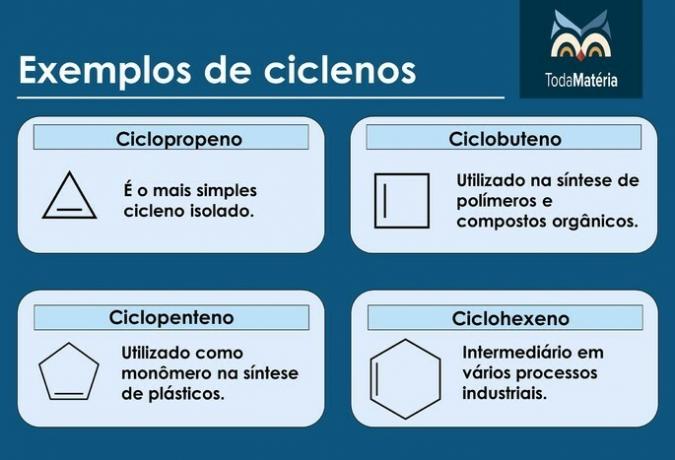
Cycles
They are closed-chain hydrocarbons with the presence of a double bond, whose structural formula is ÇnoH2n-2.
Characteristics of cyclenes
- They are also called cycloalkenes.
- Compounds of 3 to 5 carbons are unstable.
- They are usually found in natural gas, oil and petroleum.
Examples of cyclenes
cyclists
They are closed-chain hydrocarbons with the presence of a triple bond, whose structural formula is ÇnoH2n-4.
Cycling Characteristics
- They are also called cycloalkynes or cycloalkynes.
- They are cyclic and unsaturated hydrocarbons.
- They are unstable due to triple bonding and are not found in nature.
Examples of cyclines

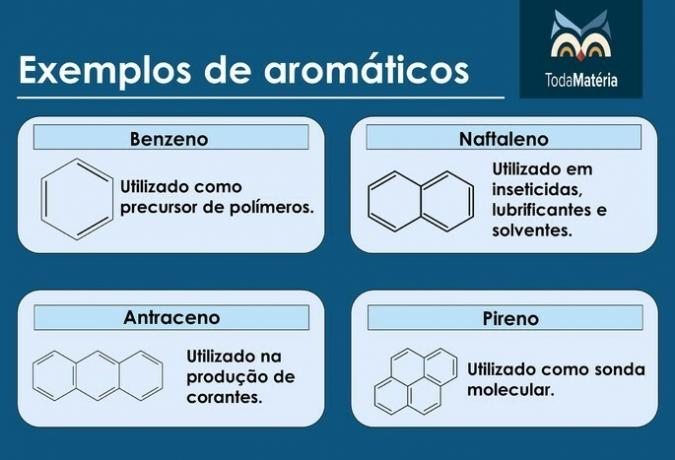
Aromatics
They are closed chain hydrocarbons with alternating single and double bonds.
Characteristics of aromatics
- They are also called arenas.
- They are unsaturated compounds, as they have 3 double bonds.
- They consist of at least one aromatic ring.
Examples of aromatics
- Petroleum
- oil refining
- Acetylene
Hydrocarbon Summary
| Occupation | General Formula |
Features |
|---|---|---|
| alkane | Open chain with simple links. |
|
| alkene | Open chain with double bond. | |
| alkyne | Open chain with triple bond. | |
| alkadiene | Open chain with two double bonds. | |
| Cyclans | Closed chain with simple connections. | |
| Cycles | Closed chain with double bond. | |
| cyclists | Closed chain with triple link. | |
| Aromatic | Variable | Closed chain with alternating single and double bonds. |
Exercises on Hydrocarbons
1. (UEMA) LPG (Liquefied Petroleum Gas), also popularly known as cooking gas, is a fuel non-renewable fossil that can run out overnight if not used with planning and without excess. It is composed, among other gases, of propane C3H8, butane C4H10 and small amounts of propylene C3H6 and butene C4H8. These organic compounds are classified as hydrocarbons that have similarities and differences between them. Based on the type of bond between carbons and the classification of the carbon chain of the compounds above, it can be stated that:
a) the unsaturated compounds are propane and butane.
b) the unsaturated compounds are propene and butene.
c) the unsaturated compounds are propene and butane.
d) the compounds have homocyclic chains.
e) the compounds have heterocyclic chains.
Alternative b) the unsaturated compounds are propene and butene.
a) WRONG. These compounds do not have unsaturations, their bonds are simple.
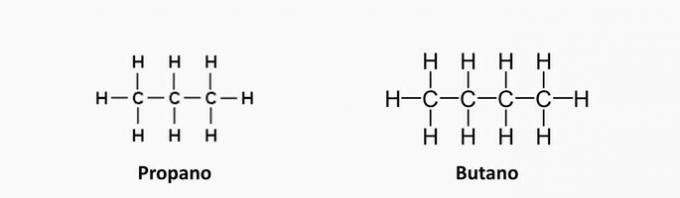
b) CORRECT. The term "en" indicates the presence of double bonds in the compounds.

c) WRONG. Butane has no unsaturations.

d) WRONG. These chains are closed and the carbon atoms are linked by single bonds.
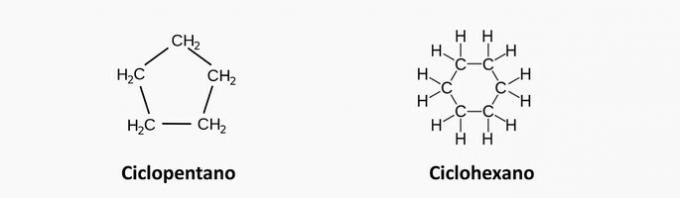
e) WRONG. These chains have a heteroatom, such as oxygen and nitrogen.

2. (Uel) One of the formula C hydrocarbons5H12 may have carbon chain:
a) saturated cyclic.
b) heterogeneous acyclic.
c) branched cyclic.
d) unsaturated open.
e) open branched.
Alternative e) open branched.
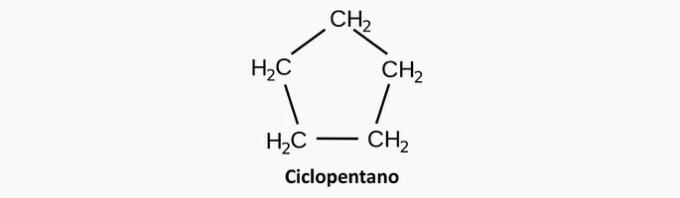
a) WRONG. A saturated cyclic compound corresponds to a cyclan, whose formula is CnoH2n.
Example:
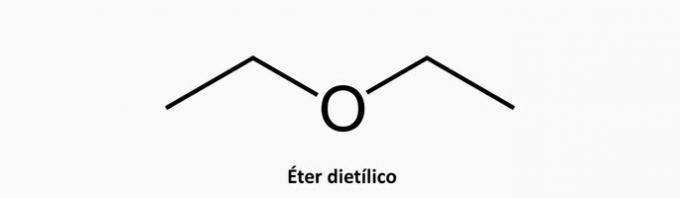
b) WRONG. A heterogeneous acyclic compound has the presence of another element in addition to the intercalated carbon in the chain.
Example:
c) WRONG. A branched cyclic compound has formula CnoH2n.
Example:
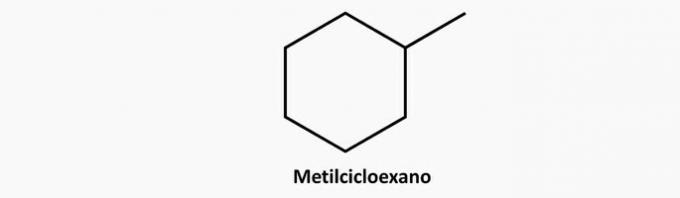
d) WRONG. An unsaturated open chain compound can be an alkene or an alkyne, the formula of which is respectively C.noH2n and CnoH2n-2.
Examples:
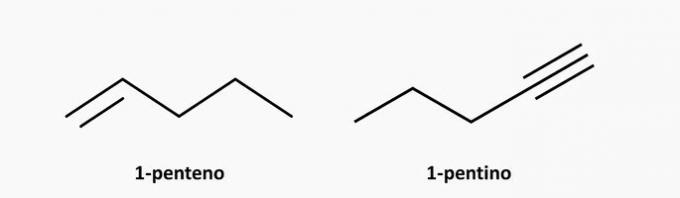
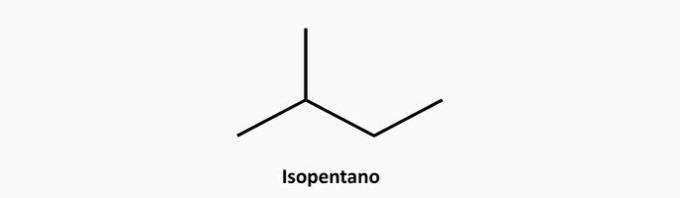
e) CORRECT. An open-chain branched compound is an alkane whose formula is CnoH2n+2. A compound of 5 carbons and 12 hydrogens could be isopentane.
Example:
3. (PUC) Alkynes are hydrocarbons:
a) saturated aliphatics.
b) saturated alicyclics.
c) double bond unsaturated aliphatics.
d) triple bond unsaturated alicyclics.
e) triple-bonded unsaturated aliphatics.
Alternative e) triple bond unsaturated aliphatics.
a) WRONG. The open-chain and single-bonded compounds are alkanes.
Example:

b) WRONG. Cyclic compounds with single bonds are cyclans.
Example:
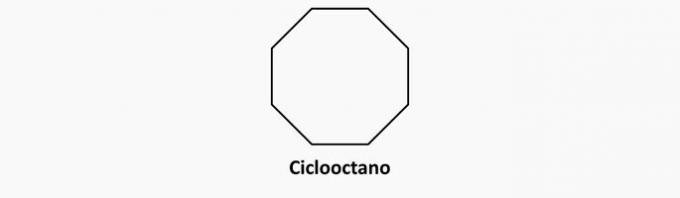
c) WRONG. Open-chain and double-bonded compounds are alkenes.
Example:
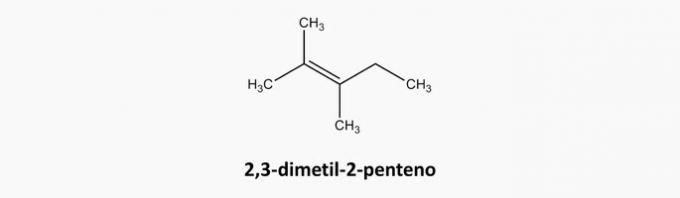
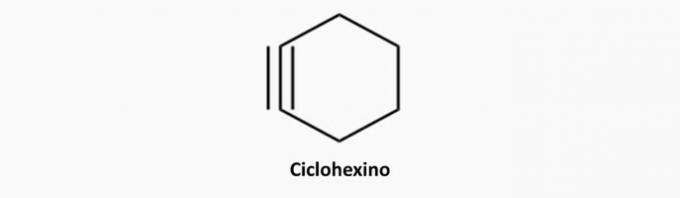
d) WRONG. Cyclic and triple-bonded compounds are cyclins.
Example:
e) CORRECT. Alkynes are open-chain and triple-bonded compounds.

Do you want to keep testing your knowledge? Be sure to check out these lists:
- Exercises on Hydrocarbons
- Exercises on Organic Chemistry
- Exercises on Organic Functions