A pure substance is formed by a single type of chemical species, that is, its composition and properties are fixed. A mix contains more than one type of component, so its organization varies.
Thus, we can only distinguish a pure substance from a mixture when we know its composition.
When comparing a glass of water and a glass of dissolved sugar, our eyes do not notice any difference. However, if we ingest the contents of the two glasses we will notice that one is the pure substance and the other is made up of a mixture.
pure substances
A pure substance is the set of just one chemical species, that is, it is not mixed with others.
Let's use water as an example. The water (H2O) is recognized for its characteristics and the specific properties of this material help us to identify it. The main water properties they are:
| Density | 1.00 g/cm3 |
|---|---|
| Fusion point | 0°C |
| Boiling point | 100°C |
When a material has fixed and invariable properties throughout its entirety, we say that it is a pure substance.
When we put table salt, sodium chloride (NaCl), into a glass of water and stir, a change will occur.

The result is a product with an intermediate density between that of water and salt. This is because water is no longer a pure substance and has become a Mix.
When trying to freeze this mixture, you will notice that the melting temperature will be less than 0 °C and that also this mixture will not boil at 100 °C, more heat will be needed to evaporate this product.
Pure simple and compound substances
Pure substances are classified as simple when in their composition there are atoms of only one chemical element.
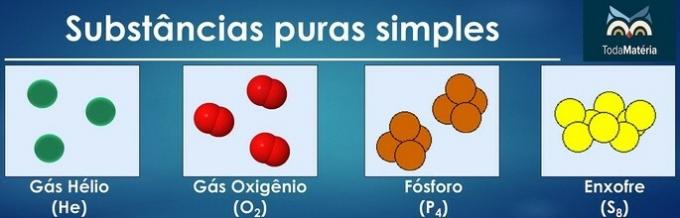
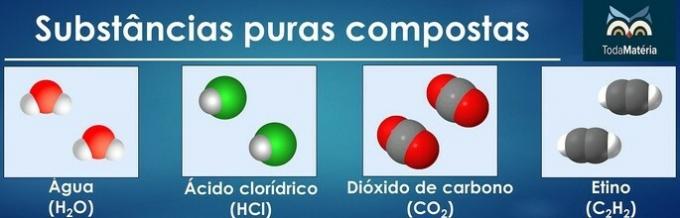
The arrangement of atoms of two or more chemical elements form pure composite substances.
Mixtures
A mixture corresponds to the joining of two or more pure substances, which are called components.
Unlike pure substances, its properties are not fixed as they depend on the proportion of components in the mixture.
See how density, a physical property, varies with the amount of salt mixed with water.
| Percentage of salt in total mass of mixture |
Mixture density (g/cm3) at 20 °C |
|---|---|
| 1 | 1,005 |
| 8 | 1,056 |
| 12 | 1,086 |
| 16 | 1,116 |
| 26 | 1,197 |
Source: FURNISS, B. S. et al. Vogel's Textbook of Practical Organic Chemistry. 4. ed. London: Longman, 1987. P. 1.312.
Therefore, the addition of water and salt, in any proportion, has a variable density and, therefore, we cannot classify the mixture as either water or salt.
Homogeneous and heterogeneous mixtures
Homogeneous mixtures are those that present the components in only one phase and, therefore, the same properties at all points.

When we visually perceive more than one phase, then the mixture is classified as heterogeneous.

Summary on pure substances and mixtures
| Pure substances and mixtures | |
|---|---|
|
homogeneous system (only one phase) |
pure substance (one component) |
|
homogeneous mixture (more than one component in the same phase) | |
|
heterogeneous system (more than one phase) |
pure substance (a component in different physical states) |
|
heterogeneous mixture (more than one component in more than one phase) |
To learn more, be sure to check these texts:
- Atoms
- Chemical elements
- Separation of mixtures
Exercises with commented feedback
1. (UFMG) A sample of a pure substance X had some of its properties determined. All alternatives have properties that are useful to identify this substance, except:
a) density.
b) sample mass.
c) water solubility.
d) boiling temperature.
e) melting temperature.
Wrong alternative: b) sample mass.
a) CORRECT. Density is the amount of matter in a given volume. As a material-specific property, it is useful for identifying a substance.
b) WRONG. Mass is the amount of matter in a body. As this property applies to any matter, regardless of its constitution, it is not possible to use it to identify a substance.
c) CORRECT. Solubility is the ability of a substance to dissolve, or not, in a given liquid. As a material-specific property, it is useful for identifying a substance.
d) CORRECT. Boiling temperature corresponds to the temperature of change from liquid to gaseous state. As a material-specific property, it is useful for identifying a substance.
e) CORRECT. Melting temperature corresponds to the temperature of change from liquid to solid state. As a material-specific property, it is useful for identifying a substance.
2. (Vunesp) The label of a bottle of mineral water is reproduced below.
| Probable chemical composition: |
|---|
| Calcium Sulfate 0.0038 mg/L |
| Calcium Bicarbonate 0.0167 mg/L |
Based on this information, we can classify mineral water as:
a) pure substance.
b) simple substance.
c) heterogeneous mixture.
d) homogeneous mixture.
e) colloidal suspension.
Correct alternative: d) homogeneous mixture.
a) WRONG. Water would be pure if its composition only had H molecules2O.
b) WRONG. A simple substance is made up of atoms of just one chemical element. Nor is pure water a simple substance, as it is formed by hydrogen and oxygen atoms (H2O) it is classified as composite.
c) WRONG. A heterogeneous mixture has more than one phase, in which case we can only observe water.
d) CORRECT. As it has only one phase, the system is homogeneous. When looking at the water bottle, we can only see the liquid, as the compounds of calcium sulfate and calcium bicarbonate are soluble in water and are therefore dissolved.
e) WRONG. A colloidal suspension is a heterogeneous mixture whose components are differentiated using a microscope.
3. (UCDB) In a Chemistry laboratory, the following mixtures were prepared:
I. water/gasoline
II. water/salt
III. water/sand
IV. gasoline/salt
V. gasoline/sand
Which of these mixtures are homogeneous?
a) None.
b) Only II.
c) II and III.
d) I and II.
e) II and IV.
Correct alternative: b) Only II.
a) WRONG. Water is an inorganic compound and gasoline an organic compound. These substances do not have the ability to interact and because they have different densities they form a heterogeneous mixture.
b) CORRECT. The salt, sodium chloride, dissolves in water forming a solution, which is a homogeneous mixture.
c) WRONG. Sand, silicon dioxide, forms a heterogeneous mixture with water.
d) WRONG. Salt is an inorganic compound and gasoline an organic compound. These substances do not have the ability to interact and because they have different densities they form a heterogeneous mixture.
e) WRONG. Sand is an inorganic compound and gasoline an organic compound. These substances do not have the ability to interact and therefore form a heterogeneous mixture.
4. (Ufes) In a well-mixed system consisting of sand, salt, sugar, water and gasoline, the number of phases is:
a) 2.
b) 3.
c) 4.
d) 5.
e) 6.
Correct alternative: b) 3.
PHASE 1: Salt and sugar are able to interact with water and through intermolecular forces the molecules bind and form a solution, which is a homogeneous mixture.
PHASE 2: Water is an inorganic compound and gasoline an organic compound. These substances do not have the ability to interact and because they have different densities they form a heterogeneous mixture.
PHASE 3: Sand is a silicate that has no chemical affinity with water and gasoline and, therefore, represents a phase.
5. (Mackenzie) The mixture formed by:
a) ice cubes and aqueous sugar solution (glucose).
b) N gases2 and CO2.
c) water and acetone.
d) water and gooseberry syrup.
e) kerosene and diesel oil.
Correct alternative: a) ice cubes and aqueous sugar solution (glucose).
a) CORRECT. It is possible to observe two phases: ice cubes and the glucose solution, so they are a heterogeneous system.
b) WRONG. Gases are always a homogeneous mixture.
c) WRONG. Hydrogen bonds form between the carbonyl of propanone and the water molecule. As they are polar substances, acetone is capable of solubilizing in water and forming a homogeneous mixture.
d) WRONG. These two components mix to form a homogeneous system, as we will only see a red liquid from the gooseberry syrup, as a dilution occurs by adding water.
e) WRONG. Both are organic compounds and due to chemical affinity they form a single phase, representing a homogeneous system.
Test your knowledge with the exercises:
- Exercises on homogeneous and heterogeneous mixtures
- Exercises on separation of mixtures
- Exercises on properties of matter


