THE octet theory was proposed by Newton Lewis, who in studies observed that atomic interaction happens so that each element acquires the electronic stability of a noble gas, that is, eight electrons in the valence layer. However, in some molecules, what is called an expansion or contraction of the octet happens, that is, the central atom establishes more or less predicted bonds.
Read too: Covalent bond - classifications and characteristics
How does octet theory work?
Of course all systems tend to look for a way to acquire as much stability as possible, and this is no different with the atom. Atoms are “base particles” of any matter and each has an electrosphere in its structure. This electrosphere was divided by Linus Pauling in energy levels and sub-levels. Pauling developed a diagram to demonstrate what the distribution of electrons around the nucleus of an atom would look like.
See the image below:
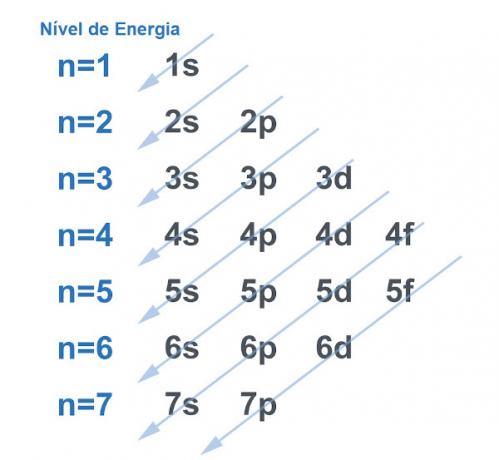
Each level and sublevel hold an amount of electrons. Making an analogy, we can say that each level is a shelf, and each sublevel is a box. In each box, fit two electrons. The atom is stable when all its electrons are paired, that is, when there are all boxes with two electrons each.
Example:
Let's do the electronic distribution of oxygen (O), which has eight electrons in its natural state.
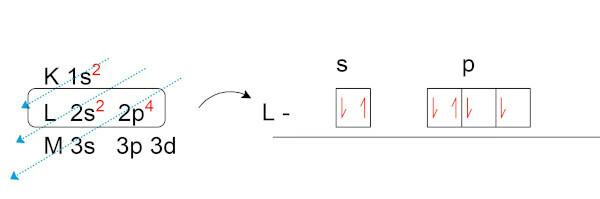
Notice that, in the valence layer (the L layer, in the p sublevel), we have two unpaired electrons. It is these electrons that establish chemical bonds with other elements looking to form electronic pairs.
Octet theory is based on the mathematics of summation of electrons. If all the sublevels of the last electron shell have two electrons each, the valence shell will have a total of eight electrons and, consequently, the atom will be stable.
Read too: Quantum numbers - numbers associated with the amount of energy of the electron
Do not stop now... There's more after the advertising ;)
noble gases
Noble gases are the only elements that can be found in nature in a monoatomic form, that is, without establishing a bond with another atom. This is because they feature electronic stability. Almost all of them have eight electrons in the valence shell., obeying the octet rule, with the exception of helium gas, which has only two electrons.

Exceptions to Octet Theory
Some compounds manage to stabilize with more or less than eight electrons in the valence shell. In these cases, exceptions to the octet theory occur.
octet expansion
It happens mainly with phosphorus (P) and sulfur (S), which are relatively large atoms and have the “d” sublevel. In this case, the atom holds more than eight electrons in its last shell.
Example:
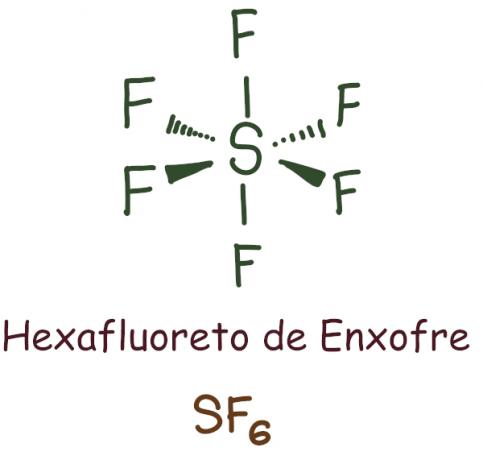
In the case of sulfur hexafluoride, the central element was left with 12 electrons, exceeding the 8 required to stabilize according to the octet rule. In this case, there was an expansion of the octet.
octet contraction
Happens with beryllium (Be), boron (B) and some oxides of nitrogen. See the example:
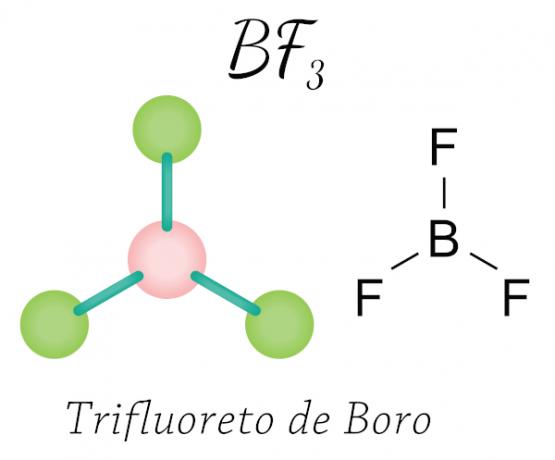
In the case of boron trifluoride, the fluorine atoms acquire the necessary eight electrons in the valence shell by sharing electronic with boron, respecting the octet rule, but the central atom (boron) does not stabilize with six electrons, thus occurring one contraction of the octet.
Also access: Classification of a sigma link: what are the criteria?
solved exercises
question 1 - (Mackenzie-SP) For sulfur and potassium atoms to acquire an electronic configuration equal to that of a noble gas, it is necessary that:
(Data: atomic number S = 16; K = 19.)
A) sulfur receives 2 electrons and potassium receives 7 electrons.
B) sulfur gives 6 electrons and potassium receives 7 electrons.
C) sulfur yields 2 electrons and potassium yields 1 electron.
D) sulfur receives 6 electrons and potassium gives up 1 electron.
E) sulfur receives 2 electrons and potassium gives up 1 electron.
Resolution
Alternative E. Sulfur is an element of column 16 or family 6A. The elements of this family tend to receive two electrons to form electronic pairs and have a total of eight electrons in the valence shell. The elements of the 1A family, which are the alkali metals, have only one electron in the valence shell. By donating this electron, the previous layer becomes the valence layer, already with the eight electrons, as dictated by the octet rule.
Question 2 - Judge the following statements as true (T) or false (F).
I ( ) The octet rule states that eight electrons are needed in the valence shell for the atom to be stable.
II ( ) The valence layer is the second electronic layer of the atom.
III ( ) Chlorine (Cl), from the halogen family, tends to gain two electrons to acquire stability.
IV ( ) Sodium (Na), an element of the 1A family, tends to lose the only electron in its valence shell.
Mark the correct alternative:
A) I, III and IV are true.
B) I and IV are true.
C) Only II is true.
D) Only the IV is false.
E) All are true.
Resolution
Alternative B. I and IV are true. Statement II is incorrect, as the valence layer is the last electronic layer of the atom, not the second. And statement III says that chlorine tends to gain two electrons, which does not check, since chlorine, being from 7A or 17 family, tends to gain only one electron to thus acquire the electronic configuration of a gas noble.
By Laysa Bernardes Marques de Araújo
Chemistry teacher


