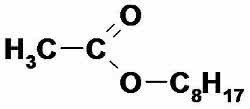
Simple substances are made up of just one chemical element; when you have two or more elements in the composition, the substances are composed.
The study of chemical decomposition reactions was important in the development of Chemistry, as they allowed the classification of substances.
Scientists observed that not all substances could be decomposed, so they used this observation to determine whether pure substances were simple or composite.
For them, compound substances divided into new substances, while simple substances did not undergo decomposition.
Although this concept is no longer accepted today, investigations carried out in this area were important for the advancement of Science.
simple substances
Simple substances are formed by just a chemical element, but the way the atoms organize to produce them can vary as follows:
- There are atoms that remain isolated.
- There are atoms that group together in different ways and can form different substances.
Example:
| isolated atom | more than one atom | |
|---|---|---|
| he | O2 | O3 |
| Helium representing both the chemical element and a helium molecule. | The joining of two oxygen atoms forms the molecular oxygen and the union of three atoms forms the ozone molecule. |
Atomicity
The number of atoms in a simple substance represents its atomicity. Therefore, there is the following classification:
- Monatomic molecules: formed by just one atom.
- Diatomic molecules: formed by two atoms.
- triatomic molecules: formed by three atoms.
Examples of simple substances

compound substances
Compound substances are also called chemical compounds, which are formed in chemical reactions by atoms or ions of different elements.
Example:
| Atoms | ions |
|---|---|
| Two simple substances (N2 and H2) react and form a compound substance (NH3). | A cation (Na+) and an anion (Cl-) react and form a compound substance (NaCl). |
As with simple substances, chemical compounds can be made up of atoms in different proportions.
This is what happens with water (H2O) and hydrogen peroxide (H2O2). Both are formed only by hydrogen and oxygen, but the number of atoms in the substances are different and, therefore, make these compounds different.
Know more about: hydrogen and oxygen.
Examples of Compound Substances
Exercises with commented feedback
1. (Cesgranrio) Identify the alternative that presents, in the sequence, the correct terms that fill in the gaps in the following statement:
"A substance _____ is formed by _____, containing only _____ of the same _____ ."
a) composite; molecules; elements; atom.
b) composite; molecules; atoms; element.
c) chemistry; elements; molecules; atom.
d) simple; atoms; molecules; element.
It's simple; molecules; atoms; element.
Correct alternative: e) simple; molecules; atoms; element.
a) WRONG. A compound substance is a molecule when in its composition there are atoms of different chemical elements.
b) WRONG. A compound substance is formed by atoms of different chemical elements and not the same element.
c) WRONG. Molecules are groups of atoms of elements joined by covalent bonds.
d) WRONG. Molecules are formed by atoms of chemical elements.
e) CORRECT. "A substance simple is formed by molecules, containing only atoms of the same element.”
1) Air is a homogeneous material made up of simple substances.
2) Of the substances alcohol, gold, diamond and acetone, only gold is a simple substance.
3) By measuring melting and boiling temperatures, it is possible to distinguish between simple and composite substances.
4) Material separation processes are used to obtain simple substances from composite substances.
1) WRONG. Air is a mixture of gases, but not all are simple substances.
The simple substances present in the air are: nitrogen, oxygen and noble gases. However, there is also water vapor and carbon dioxide, which are composite substances.
2) WRONG. Both alcohol and acetone are composite substances, classified as organic compounds. Gold and diamond are simple substances.
Alcohol is a chemical compound whose general formula is R-OH. Acetone is the trade name for propanone, a compound that has the formula CH3(CO)CH3.
Gold represents the chemical element with the symbol Au and is a metal of great value found in nature in the form of nuggets. Diamond is a crystal composed only of carbon atoms.
3) WRONG. Melting and boiling points are physical properties of matter, used to distinguish a material from a substance.
4) WRONG. In a separation process, both simple and composite substances can be obtained.
Example:
Pyrolysis of water produces two simple substances: hydrogen and oxygen.
Photolysis of hydrogen peroxide produces a compound and a simple substance: water and oxygen.
See too: Organic Functions
3. (Mackenzie) The number of simple substances among the formula substances: O3, H2O, Na, P4, CH4, CO2 and Co is:
a) 2.
b) 3.
c) 4.
d) 5.
e) 7.
Correct alternative: c) 4.
Altogether, four simple substances appear in the statement, while the others are composed.
Simple substances:
- O3: Ozone molecule formed by atoms of the element oxygen.
- Na: Isolated atom of the element sodium.
- P4: Phosphorus molecule composed of four atoms of the element.
- Co: Isolated atom of the cobalt element.
Compound Substances:
- H2O: Water is made up of atoms of two elements: hydrogen and oxygen.
- CH4: Methane is formed by atoms of two elements: carbon and hydrogen.
- CO2: Carbon dioxide is formed by atoms of two elements: carbon and oxygen.
See too: Chemical bonds
4. (UFPA) Considering the reaction:
Among reagents and products are present:
a) 2 simple substances and 2 compounds.
b) 1 simple substance and 3 compounds.
c) 3 simple substances and 1 compound.
d) 4 simple substances.
e) 4 compound substances.
Correct alternative: a) 2 simple substances and 2 compounds.
simple substances:
- C: Isolated atom of the element carbon.
- H2: Molecule formed by atoms of the element hydrogen.
compound substances:
- H2O: Water is made up of atoms of two elements: hydrogen and oxygen.
- CO: Carbon monoxide is formed by atoms of two elements: carbon and oxygen.
See too: Mixtures
5. (UFRGS) Granite consists of four minerals: feldspar, magnetite, mica and quartz. If one of these minerals can be separated from the others, it can be said that granite is:
a) an element
b) a simple substance
c) a compound substance
d) an ionic compound
e) a mixture
Correct alternative: e) a mixture.
a) WRONG. Granite is formed by chemical compounds called oxides. Oxides have different chemical elements, such as silicon and aluminum, combined with oxygen.
b) WRONG. A simple substance is made up of atoms of just one element. Granite is formed from the combination of elements in different proportions.
c) WRONG. The composition of granite is variable and has several composite substances. The most common are: SiO2, Al2O3,K2o, na2O, CaO, FeO and Fe2O3.
d) WRONG. Ionic compounds have their atoms joined by ionic bonds. Oxides, an inorganic function present in granite, are binary compounds formed by covalent bonds, in which electrons are shared.
e) CORRECT. Granite is a mixture of rock-shaped minerals. Its constituents are:
- Feldspar: mineral composed of aluminum silicate that contains different proportions of calcium, potassium and sodium. Its chemical formula is: (K, Na, Ca) (Si, Al)4O8.
- Magnetite: mineral formed by iron II and iron III oxides: Its chemical formula is: FeO.Fe2O3.
- Mica: mineral formed by hydrated silicates of aluminum, potassium, sodium, iron, magnesium and lithium. An example of mica is: KAl2[Si3Hello10](OH, F2)).
- Quartz: mineral formed by silicon dioxide. Its chemical formula is: SiO2.
You may also be interested in: Subject: what is it, composition and examples.