Chromatography is a process of separating and identifying components of a mixture.
This technique is based on the migration of compounds from the mixture, which have different interactions through two phases.
- mobile phase: phase in which the components to be isolated "run" through a fluid solvent, which can be liquid or gas.
- stationary phase: fixed phase in which the component being separated or identified will fix on the surface of another liquid or solid material.
To understand chromatography, you need to know two basic concepts:
- elution: is the chromatographic run.
- eluent: it is the mobile phase, a type of solvent that will interact with the samples and promote the separation of the components.
The chromatographic process consists of passing the mobile phase over the stationary phase, inside a column or on a plate. Thus, the components of the mixture are separated by the difference in affinity across the two phases.
Each of the components of the mixture is selectively retained by the stationary phase, resulting in differential migrations of these components.
Chromatography serves for substance identification, compound purification and separation of components from mixtures.
 With chromatography it is possible to separate the ink components from pens
With chromatography it is possible to separate the ink components from pens
Check out how to do this experiment at: Chemistry Experiments
Types of Chromatography
Chromatography types are divided by the following criteria:
Physical form of the chromatographic system:
1. Column chromatography
Column chromatography is the oldest chromatographic technique. It is a technique for separating components between two phases, solid and liquid, based on the ability to adsorption and solubility.
The process takes place in a column of glass or metal, usually with a tap at the bottom. The column is filled with a suitable adsorbent that will allow solvent flow.
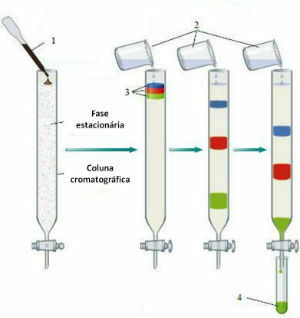 Column chromatography
Column chromatography
The mixture is then loaded onto the column with a less polar eluent. A continuous sequence of several eluents is used in order to increase its polarity and, consequently, the dragging power of more polar substances.
Thus, the different components of the mixture will move at different speeds, depending on their affinity with the adsorbent and eluent. This makes the separation of components possible.
2. planar chromatography
Planar chromatography comprises paper chromatography and thin layer chromatography:
- Chromatography on paper: is a technique for liquid-liquid, in which one of them is fixed to a solid support. It receives this name because the separation and identification of the mixture components takes place on the surface of a filter paper, which is the stationary phase.
- Thin layer chromatography: is a technique for liquid-solid, in which the liquid phase rises through a thin layer of adsorbent on a support, usually a glass plate placed inside a closed container. When ascending, the solvent will drag more compounds that interacted less in the stationary phase. This will cause the more adsorbed components to separate.
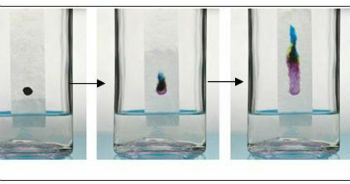 Chromatography on paper
Chromatography on paper
Mobile phase employed:
1. Gas chromatography
Gas chromatography is a process of separating the components of the mixture through a mobile gas phase over a solvent.
This method takes place in a narrow tube, through which the components of the mixture will pass through a stream of gas, which represents the mobile phase, in a column-type flow. The stationary phase is represented by the tube.
The factors that promote the separation of the components are: the chemical structure of the compound, the stationary phase and the column temperature.

Gas chromatography steps
2. liquid chromatography
In liquid chromatography, the stationary phase is made up of solid particles arranged in a column, which is traversed by the mobile phase.
Liquid chromatography comprises classical liquid chromatography and high performance liquid chromatography:
- Classic liquid chromatography: the column is usually filled only once, as part of the sample usually adsorbs irreversibly.
- High performance liquid chromatography: is a technique that uses high pressure pumps to elute the mobile phase. This allows the mobile phase to migrate at a reasonable speed through the column. Thus, you can carry out the analysis of several samples in a short time. However, it needs specific equipment.

Liquid Chromatography Steps
3. supercritical chromatography
Supercritical chromatography is characterized by using a pressurized vapor in the mobile phase, above its critical temperature.
The most used supercritical eluent is the carbon dioxide.
Stationary phase employed:
Depending on the stationary phase used, chromatography can be liquid or gas:
- liquid stationary phase: the liquid is adsorbed onto a solid support or immobilized on it.
- Solid stationary phase: when the fixed phase is a solid.
Read too:
- Separation of Mixtures
- Homogeneous and heterogeneous mixtures
- Solute and Solvent
- chemical solutions
- Solubility



