If soap is made from oils and grease, how is it able to clean greasy surfaces? It's a curious question, since the basic composition of soaps is precisely animal fats. and vegetable oils, all insoluble in water, hence why water alone does not clean these compounds.
A great discovery revolutionized cleaning, through which it is possible to clean accumulated dirt. Everything is very simple: the mixture of oils (esters) with alkaline solutions (sodium or potassium hydroxide) gave rise to a product that dissolves in water and removes fat. We're talking about soap, follow the equation that made its production possible:
Oil + base → soap + glycerol
Returning to the previous question, if oils are insoluble in water, how can they be removed using soap and water? Thanks to the soap's polar and non-polar character. Below is a demonstration of how the interaction between soap, water and oil works.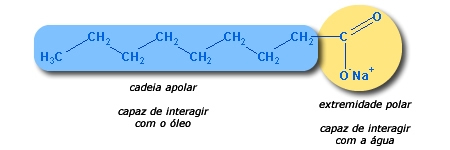
Note that the soap molecule has a polar part and a non-polar part. The nonpolar chain formed by hydrocarbons (−
Thus it is possible to form an emulsion (mixture) characterized by the foam. It is from this interaction between the components of the soap that it is possible to clean surfaces full of grease.
By Líria Alves
Graduated in Chemistry
Brazil School Team
See more!
Soap or washing, which came first?
Turning used cooking oil into soap.
Chemistry Curiosities - Chemistry - Brazil School
Source: Brazil School - https://brasilescola.uol.com.br/quimica/como-sabao-limpa.htm
