There are several ways to represent the same chemical compound, this is done through the Chemical Formula, which can provide us with some information about the substance it represents.
We will now know 5 forms of presentation of a compound, taking as an example the organic substance Benzene.
flat structural formula: It is the formula that describes all the elements present in the compound molecule and the arrangement of the atoms through of a flat representation, the pairs of electrons that establish the chemical bond are represented by dashes. (─). The structural formula above shows how the atoms that make up Benzene are linked together.

molecular formula: In this type of formula, the element is represented by its symbol followed by the index (subscript number) indicating the number of atoms present in each molecule. So we have the number of atoms that make up the substance, as we see, there are 6 Carbons (C) and 6 Hydrogens (H) in a Benzene molecule.

empirical formula: it's like a “summarized” way of representing the compounds. The simple structure only allows us to understand the shape of the Benzene structure and the presence of unsaturations (double bonds).

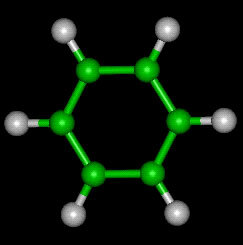
Ball Structure Formula: in this way carbons and hydrogens are identified by color and size, where C are represented by the black color and the H by the white color.


Formula in stroke and ball structure
This last representation is the most used to insert the Organic Chemistry subject, it allows observe the structure's shape and still visualize the type of bond that joins the hydrogens to the chain main.
In fact, there are parts on the market for assembling the structure of lines and balls, as if it were a puzzle, where the teacher can demonstrate step by step the formation of organic compounds.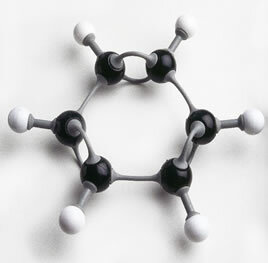
Photo of benzene ring assembled with plastic parts: an excellent teaching material to be worked on in the classroom.
By Líria Alves
Graduated in Chemistry
Brazil School Team
See more!
Structural formulas of carbon
chemical formulas
General chemistry - Chemistry - Brazil School
Source: Brazil School - https://brasilescola.uol.com.br/quimica/classificacao-das-formulas.htm

