Salts are chemical substances formed by ionic bonds between atoms. The inorganic salt function corresponds to ionic compounds that have at least one cation other than H+ and an anion other than OH-.
Salts are present in our daily lives, being widely used in food and also in other areas. They are examples of salts:
- Sodium chloride (NaCl): popularly known as table salt
- Calcium carbonate (CaCO3): present in marbles and limestone
- Calcium Sulfate (CaSO4): makes up school chalk and plaster
- Sodium Bicarbonate (NaHCO3): used in cooking, medicine and as a cleaning agent
These substances are usually formed in a neutralization reaction, when an acid and a base react to produce a salt and water.
HCl(acid) + NaOH(base) → NaCl(salt) + H2O(Water)
In this reaction, the reagents hydrochloric acid (HCl) and sodium hydroxide (NaOH) form the products sodium chloride (NaCl) and water (H2O).
In ionic bonding occurs with the transfer of electrons between atoms and for this one of the atoms must be a metal and the other a non-metal. With this, positive chemical species, the cations, are formed by donating electrons and the negatively charged anions, which received them.
Observe in the image below how sodium chloride (NaCl) is formed.
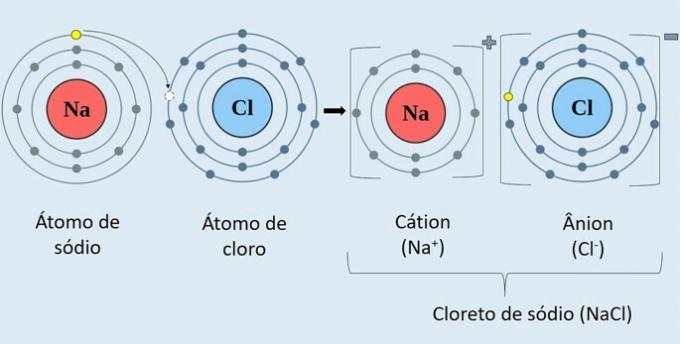
The sodium metal atom (Na) donates an electron to the chlorine atom (Cl). Thus, the Na cation is formed+, which consists of the positive sodium ion, and the Cl anion-, which corresponds to the negative chlorine ion.
know more about acids and bases.
Characteristics of Salts
It is important to note that, in aqueous solution, acids always release H cation+ and the bases release the OH anion- (concept of Arrhenius).
Salts, however, do not always have the same cation or anion and, for this reason, do not show well-defined functional properties. However, we can say that in general:
- They are ionic compounds (formed by clusters of ions and not molecules);
- Many have a characteristic salty flavor (almost always poisonous);
- They are solid and crystalline;
- Conduct electric current in solution;
- They melt and boil at high temperatures;
- Soluble in water (exceptions: some sulphides; the chlorides, bromides and iodides with the Ag cations+, hg22+ and Pb2+, between others).
Also read about chemical functions.
Classification and nomenclature of salts
According to the way the salt formation reaction occurs, they are classified into three types:
Neutral or normal salts
Total neutralization reaction (all H react+ of acid and all OH- of the base). These salts when dissolved in water do not change the pH.
Examples:
NaOH (base) + HCl (acid) → NaCl (normal salt) + H2O
3NaOH (base) + H3DUST4 (acid) → Na3DUST4 (normal salt) + 3H2O
Name of Normal Salts: the name of the salt comes from the name of the acid anion, whose termination _hydric or _oso or _ico will be replaced respectively by: _etho ou_ito or _act and the base cation.
Salt = (anion name) + suffix etho/ito/actin (cation name).
Thus:
- acid chlorinehydric (HCl) + hydroxide of sodium (NaOH) → chlorineethosodium (NaCl) + water
- acid nitratebone(HNO2) + hydroxide of potassium(KOH) → nitratevery of potassium(KNO2) + water
- acid orthophosphorich (2h3DUST4) + hydroxide of calcium(3Ca(OH)2 → orthophosphact of calcium [Here3(DUST4)2] + water (6H2O)
Acid salts or hydrogen salts
Partial acid neutralization reaction (when not all H+ of the acid react, so the salt has in its structure one or more ionizable hydrogens from the acid).
Example:
NaOH (base) + H2ONLY4 (acid) → NaHSO4 (acid salt) + H2O
Name of Acid Salts: similar to normal salts, but with indication of H number+ by prefixes mono, di, tri, etc.
Sal = H number prefix+ + (anion name) + suffix etho/ito/actin (cation name).
acid sulfurich (H2ONLY4) + hydroxide of sodium(NaOH) → monohydrogensulphuractin sodium (NaHSO4) + water
acid orthophosphorich (H3DUST4) + hydroxide sodium(NaOH) → dihydrogen-orthophosphact sodium (NaH2DUST4) + water
Basic salts or hydroxy salts
Partial base neutralization reaction (If not all hydroxyls react, the salt has one or more hydroxyls in its structure).
Example:
Ca(OH)2 (base) + HCl (acid) → Ca (OH)Cl (basic salt) + H2O
Name of Basic Salts: similar to normal salts, but indicating the OH number- in its structure.
Sal = OH number prefix- + (anion name) + suffix etho/ito/actin (cation name).
acid chlorinehydric (HCl) + hydroxide of calcium [Ca(OH)2] → monohydroxychlorineetho of calcium [Ca(OH)Cl] + water
acid chlorinehydric (2HCl) + hydroxide aluminum [Al(OH)3] → monohydroxychlorineethoin aluminum [Al(OH)Cl2] + water
Double or mixed salts
Reaction of a di, tri or tetrabase with different bases (double salt for the cation) or of a di, tri or tetrabase with different acids (double salt for the anion).
Examples:
As for the cation:
H2ONLY4 (diacid) + KOH (base) + NaOH (base) → KNaSO4 (double potassium and sodium sulfate) + 2H2O
H3DUST4 (triacid) + 2KOH (base) + NaOH (base) → K2NaPO4 (monosodium dipotassium orthophosphate)
As for the anion:
Ca(OH)2 (dibase) + HBr (acid) + HCl (acid) → CaBrCl (calcium chloride-bromide) + 2H2O
Al(OH)3 (tribase) + H2ONLY4(acid) + HCl (acid) → Al (SO4)Cl (aluminium chloride sulfate) + 3H2O
Know the main inorganic functions and be sure to check entrance exam questions on the subject, with commented resolution, in: exercises on inorganic functions.



