Carbon monoxide is a colorless, odorless, flammable and toxic gas.
Its molecular formula is CO. It consists of a carbon and oxygen molecule.
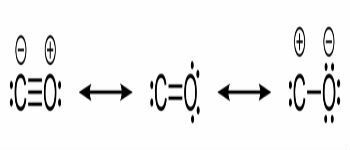
Structural formulas of carbon monoxide and chemical bonds between carbon and oxygen
It originates in two ways:
- Natural broadcast sources: activity of volcanoes, natural gas and electrical discharges.
- human activities: result of combustion incomplete fossil fuels. Burning produces tons of CO, being one of the main activities that release CO into the atmosphere.
Features
Carbon monoxide belongs to the group of oxides. It is classified as neutral oxide, those formed by a metal plus oxygen. Furthermore, it does not react with water, acids and bases.
It is used as a reducing agent, it removes oxygen from a compound and produces the carbon dioxide (CO2). Therefore, its use in the processing of ores, such as iron, is very common. As well as in the production of organic substances, such as acetic acid, plastics, methanol, among others.
When reacting with oxygen in the air, it produces carbon dioxide. According to the following chemical reaction:
2 CO + O2 → 2 CO2In surface waters, the high concentration of carbon monoxide serves as an energy source for microorganisms.
Carbon monoxide is one of the gases of greenhouse effect. Its concentration in the atmosphere contributes to greater heat retention. It is therefore considered a polluting gas.
Intoxication
CO has a high affinity for hemoglobin. As it is toxic, its inhalation causes effects on human health and can even lead to death.
Because CO has no odor, it may be being inhaled unnoticed. Therefore, in some cases, the person takes a long time to notice the intoxication.
When inhaled at low concentration, it causes migraines, slow thinking, eye irritation and loss of manual ability. In high concentrations it can cause seizures, loss of consciousness and even death from suffocation.
But how does intoxication happen?
THE hemoglobin connects, of course, to the O2 and transports it to body tissues. However, the affinity between CO and hemoglobin is much greater, about 250 times more than with O2.
In the presence of CO, hemoglobin binds to it, preventing the transport of oxygen to cells. The combination of CO with hemoglobin gives rise to carboxyhemoglobin.
The main causes of CO poisoning occur in the following situations:
- Car engines running indoors;
- Burn of natural gas in inefficient heaters;
- Gas exhaust from kitchen or wood oven, in poorly ventilated areas.
In countries with a cold climate, houses tend to stay closed longer and use heating systems. To avoid gas accidents, CO detectors are increasingly used.
Learn more, read also:
- carbon cycle
- Carbon
- Fossil fuels
- Oxygen



