THE molecular geometry, that is, the arrangement of the atoms of a molecule, can be determined by the chemist's rule Gillespie, in which he lists the number of atoms attached to a central atom and the number of clouds electronics.
Knowledge about the geometry of a molecule is extremely important because it helps us to determine the polarity and, consequently, the solubility (according to the rule of similar dissolves similar).
with the call tetrahedral geometry, is no different. See the criteria for determining it according to Gillespie's rules:
Pentatomic molecules (five atoms);
Absence of electronic clouds in the central atom;
composite molecules or compound anions.
Some examples of molecules whose geometry is tetrahedral they are:
CH4

CH structural formula4
Carbon has four electrons in its valence shell and all these electrons bond with hydrogens. That's why carbon doesn't have an electronic cloud (extra pair of electrons).
ONLY4-2
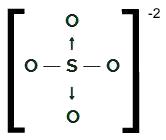
OS structural formula4-2
Sulfur, which has six electrons in the valence shell, makes two single bonds with two oxygen atoms and two
dative covalent bonds with the other two oxygens. In single bonds, it uses two of its electrons and, in each dative, it uses another two (total of four electrons involved in datives), totaling six electrons. As such, it has no cloud left.NH4+

NH structural formula4+
Nitrogen, which has five electrons in the valence shell, makes three single bonds with the hydrogen atoms and a dative bond with the other hydrogen. In single bonds, it uses three of its electrons and, in dative, it uses another two, totaling five electrons. As such, it has no cloud left.
CH3Cl
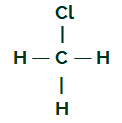
CH structural formula3Cl
Do not stop now... There's more after the advertising ;)
Carbon has four electrons in its valence shell and all these electrons are bonding with the hydrogens and the chlorine atom. For this reason, carbon does not have an electronic cloud.
→ Molecule Polarity
Knowing that a given molecule has tetrahedral geometry, we can determine its polarity of extremely simple form, since the molecule has its four poles occupied (four ligands in the atom central). For this, it is enough to know the characteristics of the binders and consider one of the following rules:
If the number of clouds is equal to the number of equal ligands = nonpolar molecule;
If the number of clouds is different from the number of equal ligands = polar molecule.
In the following two molecules, whose geometry is tetrahedral, we can apply the rules above and indicate their polarity:
Methane Molecule
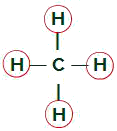
Equal ligands in the Methane molecule
The methane molecule has four electron clouds (four single bonds) and also four hydrogen atoms attached to the central atom. Thus, we havefourclouds and four equal binders, soon, the molecule is non-polar.
Observation: If the substance molecule is apolate, it dissolves well into another substance whose molecules are also non-polar.
Chloromethane Molecule

Equal ligands in the chloromethane molecule
The chloromethane molecule has four electron clouds (four single bonds) and three hydrogen atoms and one chlorine atom attached to the central atom. Thus, we havefourclouds and three equal ligands (three hydrogens); soon, the molecule is polar.
Observation: If the substance molecule is polar, it dissolves well into another substance whose molecules are also polar.
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Tetrahedral geometry"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/geometria-tetraedrica.htm. Accessed on June 28, 2021.