Since the oses or monosaccharides are carbohydrates (carbohydrates) that have in their structure two different organic groups: alcohol and ketone or alcohol and aldehyde; it can happen that the oxygen in the ketone or the aldehyde reacts with some hydroxyl group characteristic of alcohols, causing an intramolecular interaction.
For example, the following glucose molecule is an aldose, that is, it has the aldehyde group and not the ketone group. Its aldehyde group reacts with the hydroxyl of carbon 4, which cyclizes the molecule and creates a ring with 5 atoms, 4 carbon and 1 oxygen; therefore, it is considered a pentanel.

Cycling of glucose, with formation of glucofuranose.
This compound formed is called furanose, furanoic or furanose. This is because its structure looks a lot like the structure of a furan (C4H4O), shown below:
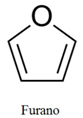
Furan structure.
Glucose cyclization can also occur with the 5 hydroxyl carbon, generating a hexanel.
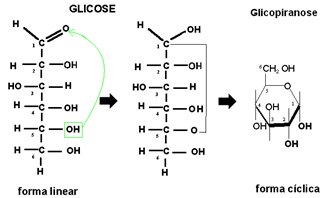
Cycling of glucose, with formation of glycopyranose.
This cyclic form of glucose is called Ose. pyranoic, pyranoic or pyranose; because it looks a lot like pirano:
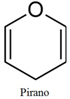
Pyranus structure.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/ciclizacao-das-oses.htm


