THE Organic chemistry is a part of Chemistry that each year has been gaining prominence in entrance exams and in Enem, especially the subject organic reactions. With that in mind, below, you will have access to fundamental tips on organic reactions to facilitate your studies!
1st) How to recognize a combustion
Combustion reaction is one in which any organic compound (such as methane) reacts with oxygen gas to form water and a carbon gas (such as carbon dioxide).
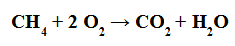
Equation representing the combustion of an organic compound
2nd) What are the products originated in a combustion reaction?
The products originated in a combustion reaction depend on the type of combustion:
Complete combustion: is the one in which we have the formation of water and carbon dioxide (CO2) .
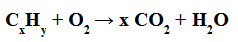
Example of a complete combustion equation
Incomplete combustion: is the one in which we have the formation of water and carbon monoxide (CO).

Example of an Incomplete Combustion Equation
3rd) What are the substances involved in a reaction of photosynthesis?
The photosynthesis reaction occurs when carbon dioxide molecules react with water molecules, in the presence of light and chlorophyll, to form carbohydrate molecules (such as glucose).
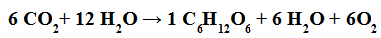
Chemical equation representing photosynthesis
Addition reactions are those in which the pi bonds in a chain are broken, and each of the carbons that were making those bonds receive new atoms.
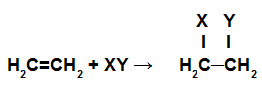
Schematic representation of an addition reaction
5th) What are the possible reactants of an addition reaction?
Organic substances that are commonly used in an addition reaction are:
alkene: Organic substance formed by only carbons and hydrogens, in an open chain with a double bond.
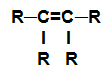
Structural formula of an alkene
NOTE: The R group can be a radical or a hydrogen element.
alkyne: Organic substance formed by only carbons and hydrogens, in an open chain with a triple bond.
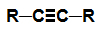
Structural formula of an alkyne
alkadiene: Organic substance formed by only carbons and hydrogens, in an open chain with two double bonds.
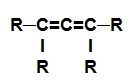
Structural formula of an alkadiene
Cyclan:Organic substance formed by only carbons and hydrogens, in a closed and saturated chain.
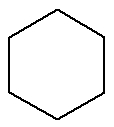
Structural formula of a cyclan
Cycle: Organic substance formed by only carbons and hydrogens, in a closed and saturated chain.
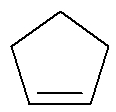
Structural formula of a cyclene
Benzene
Organic substance formed by a closed chain and composed of six carbons, three alternating double bonds and six hydrogen atoms.

Structural formula of benzene
6th) What are the possible products of an addition reaction?
The products of an addition reaction depend on the substance that is reacting with the organic compounds (alkene, alkyne, alkadiene, etc.). See the main addition reactions and their possible products:
These are reactions in which, in addition to the organic substance, one of the reactants must be hydrogen gas (H2). In this reaction, the pi bond is broken and each of the carbons that were making the bond receives a hydrogen atom. If there is more than one pi link, the process is repeated. There is the formation of a hydrocarbon.
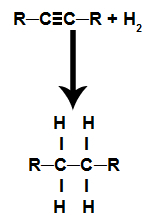
Equation representing the hydrogenation of an alkyne
These are reactions in which, in addition to the organic substance, one of the reactants must be a simple substance formed by halogen. In this reaction, the pi bond is broken and each of the carbons that were making the bond receives a halogen atom. If there is more than one pi, the process is repeated.
We will have the formation of organic halides vicinals (those in which two carbons in the chain are neighbors and contain halogen).
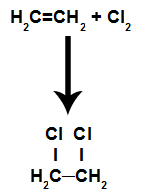
Equation representing the chlorination of an alkene
c) Addition by acid halide
These are reactions in which, in addition to the organic substance, one of the reagents must be an acid halide (HCl, HBr, HI, HCl). In this reaction, the pi bond is broken and the hydrogen in the acid goes to one carbon and the halogen goes to another. If there is more than one pi, the process is repeated. We will have the formation of a hydrocarbon.
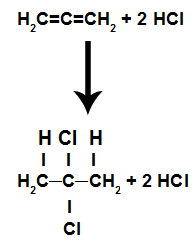
Equation representing the acid halide addition of an alkadiene
d) Hydration
These are reactions in which, in addition to the organic substance, one of the reactants must be water (H2O). In this reaction the pi bond is broken and the hydrogen in water goes to one carbon and the OH group in water goes to another carbon. If there is more than one pi, the process is repeated. We will have the formation of a hydrocarbon.
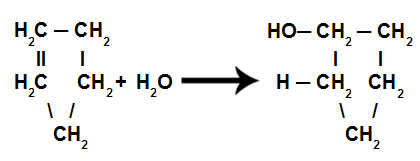
Equation representing cyclene hydration
7th) How to recognize a substitution reaction
A substitution reaction is one in which an organic compound exchanges one or more hydrogen atoms with an atom or group of the substance that is reacting with it.

Schematic representation of a substitution reaction
8th) What are the possible reactants of a substitution reaction?
The organic substances that are commonly used in a substitution reaction are:
alkane: Organic substance formed by only carbons and hydrogens, in an open and saturated chain.
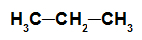
Structural formula of an alkane
Benzene
Already demonstrated in the 5th tip.
→ Benzene Derivatives: organic substances in which one or more hydrogens are exchanged for other atoms or different groups.
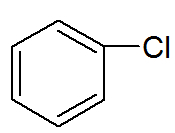
Structural formula of a benzene derivative
organic halide
Organic substance formed by atoms of carbon, hydrogen and one or more halogens (chlorine, bromine, iodine and fluorine), in an open or closed chain, saturated or unsaturated.

Structural formula of an organic halide
9th) What are the possible products of a substitution reaction
The products of a substitution reaction depend on the substance that is reacting with the organic compounds. See the main replacement reactions and their possible products:
a) Halogenation
These are reactions in which, in addition to the organic substance, one of the reactants must be a simple substance formed by halogen.
In this reaction, a hydrogen in the organic compound is replaced by a halogen atom, forming an acid halide and an inorganic acid.

Equation representing the chlorination of an alkane
Reaction in which the organic compound reacts with nitric acid (HNO3). In this reaction, the compound loses a hydrogen and receives the nitro group (NO2), resulting in a nitro compound and water, regardless of which organic compound has reacted with the acid.
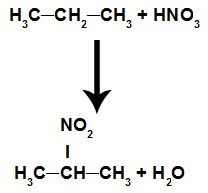
Equation representing alkane nitration
Reaction in which the organic compound reacts with sulfuric acid (H2ONLY4). In this reaction, the compound loses a hydrogen and receives the sulfonic group (SO3H), resulting in a sulfonic acid and water, regardless of which organic compound has reacted with the acid.

Equation representing benzene sulphonation
Reaction in which the organic compound reacts with an organic halide. In this reaction, the organic compound loses a hydrogen and receives the radical that was attached to the halogen in the organic halide. The reaction results in hydrocarbon and an inorganic acid, regardless of which organic compound has reacted with the acid.

Equation representing the alkylation of benzene
Reaction in which the organic compound reacts with a acid halide.
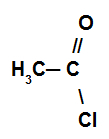
Example of an acid halide
In this reaction, the organic compound loses a hydrogen and receives the acyl group from the acid halide.
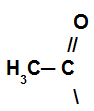
Representation of the acyl group of the acid halide
The reaction results in a ketone and an inorganic acid, regardless of which organic compound has reacted with the acid.
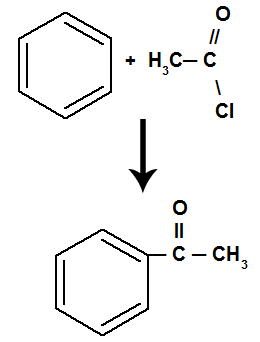
Equation representing the acylation of benzene
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/dicas-fundamentais-sobre-reacoes-organicas.htm

