Protein structure refers to its natural conformation necessary to perform its biological functions.
Proteins are macromolecules formed by the union of amino acids.
Amino acids are linked together by peptide bonds. Molecules resulting from the joining of amino acids are called peptides.
Proteins have four structural levels: primary, secondary, tertiary and quaternary structure.
Primary structure of proteins
The primary structure corresponds to linear amino acid sequence joined by peptide bonds.
In some proteins, replacing one amino acid with another can cause illness and even death.
Spatial structures of proteins
The spatial structures of proteins result from the folding and folding of the protein strand on itself.
The functional properties of proteins depend on their spatial structure.
Secondary Structure
The secondary structure corresponds to the first level of helical winding.
It is characterized by regular and repetitive patterns that occur locally, caused by the attraction between certain nearby amino acid atoms.
The two most common local arrangements that correspond to secondary structure are alpha-helix and beta-leaf or beta-pleated.
- alpha helix conformation: characterized by a three-dimensional arrangement in which the polypeptide chain assumes helical conformation around an imaginary axis.
- beta sheet conformation: occurs when the polypeptide chain extends in zig-zag and can be arranged side by side.
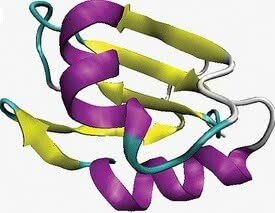
Secondary structure. In purple the alpha-helix conformation and in yellow the beta-leaf
Tertiary Structure
The tertiary structure corresponds to the folding of the polypeptide chain on itself.
In tertiary structure, the protein takes on a specific three-dimensional shape due to the global folding of the entire polypeptide chain.
Quaternary Structure
While many proteins are formed by a single polypeptide chain. Others are made up of more than one polypeptide chain.
The quaternary structure corresponds to two or more polypeptide chains, identical or not, that group and fit together to form the total structure of the protein.
For example, the insulin molecule is made up of two interconnected chains. Meanwhile, hemoglobin is made up of four polypeptide chains.
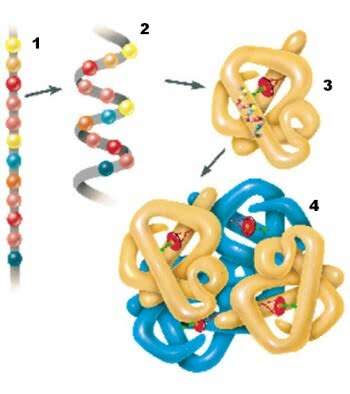
1. Primary structure; 2. Secondary structure; 3. Tertiary structure; 4. Quaternary structure.
Learn more about Proteins.
Protein Denaturation
In order to perform their biological functions, proteins need to present their natural conformation.
Heat, acidity, salt concentration, among other environmental conditions can alter the spatial structure of proteins. As a result, their polypeptide chains unwind and lose their natural conformation.
When this occurs, we call it protein denaturation.
The result of denaturation is the loss of the biological function characteristic of that protein.
However, the amino acid sequence is not altered. Denaturation only corresponds to the loss of spatial conformation of proteins.
To learn more, read also about peptides and peptide bonds.