Benzene is an aromatic hydrocarbon whose formula is C6H6.
It is a colorless liquid compound with a characteristic sweet smell and highly toxic. Benzene inhalation can cause serious health problems.
All aromatic hydrocarbons have benzene or aromatic rings.
Features
Benzene was discovered in 1825 by scientist Michael Faraday (1791-1867).
Scientists have long tried to understand the structure of benzene.
Only in 1865, the chemist Kekulé (1829-1896) proposed the shape of a hexagonal ring, with a pair of balanced structure and alternating double bonds.
The ability to move or electronically relocate gives benzene its aromatic character.
Other important characteristics of benzene are:
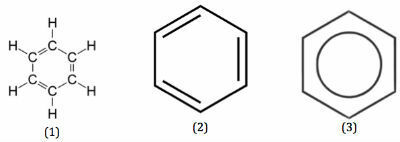
- Hexagon-shaped and closed structure.
- It is composed of six carbon atoms that are equivalent and equidistant. This is because their monosubstituted derivatives are the same in totality.
- Its disubstituted derivatives result from three different isomers.
Benzene can be represented through the following three structures:
Learn more about the Aromatic Hydrocarbons.
Applications and toxicity
Benzene is the aromatic hydrocarbon present in Petroleum, in gasoline and cigarette smoke. It can also be found in volcanoes and forest fires.
In industries and laboratories it is used as a solvent and is an important raw material for the manufacture of other products.
Despite its importance in the chemical and industrial fields, benzene is highly harmful to humans.
Benzene inhalation is the main form of intoxication. In a short period it can cause tremor, drowsiness, rapid heart rate and unconsciousness.
Already ingesting food contaminated by benzene can lead to death.
Also, benzene is considered a carcinogen.
Learn more, lhey also:
- Hydrocarbons
- alkanes
- waves
- Alkynes
- Butane
Curiosity
Chemist Kekulé discovered the structure of benzene after having a dream in which he saw the structure as a snake swallowing its tail.