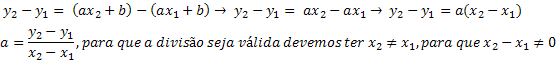The elements named after alkaline earth metals are those that belong to family 2 or IIA, of the Periodic Table. Such group comprises the following chemical elements: Be (Beryllium), Mg (Magnesium), Ca (Calcium), Sr (Strontium), Ba (Barium) and Radium (Ra).

They have two electrons in the valence shell, and their generic distribution is represented by ns2, where no represents the number of the last level. Due to this characteristic, they tend to lose these two electrons, giving rise to 2+ charge cations.
The term “earth” refers to “existing on earth”, as this is where these elements are found, mainly in minerals. For example, calcium, in the form of the calcium phosphate compound (Ca3(DUST4)2), is found in the earth's crust and serves as a raw material for the production of phosphorus. It is also present in the bones of animals, so much so that when these are calcined, the so-called “bone meal” is obtained, which contains calcium phosphate.
Among the alkaline earth metals, the ones that stand out the most are the Calcium it's the Magnesium.
- Calcium
The calcium ion (Ca2+) is present in about 90% of our bones and teeth; and in animals it is also present in their shells. Its function is to strengthen the bone structure and serve as a muscle relaxant. Lack of calcium in the body can cause muscle cramps, tetanus and osteoporosis. That's why it's important to eat it through foods rich in calcium, such as milk and its derivatives (butter, cheese, etc.), meat and vegetables.
On the other hand, too much calcium can cause muscle relaxation, kidney stones, and bone pain.
In addition to the aforementioned foods, calcium is still present in many parts of nature, especially in minerals such as calcite (CaCO3) or calcium carbonate, which is a constituent of limestone and marble, and marble is used in the manufacture of sinks, statues, floors and staircases. Calcium carbonate is also the builder of stalactites and stalagmites in caves. Calcium is also present in natural gypsum (CaSO4. 2 hours2O), called gypsum. This salt can be dehydrated, giving rise to CaSO4 anhydrous, which is school chalk.
In industry, virgin lime (CaO) is produced, slaked lime, also called hydrated lime or slaked lime, which is actually calcium hydroxide (Ca (OH)2), used in whitewashing paints and in the preparation of mortar. The gentle heating of the gypsum allows obtaining the hemihydrated calcium sulfate, which is gypsum, used in construction in the production of molds in Dentistry and Medicine to protect bone fractures, such as the one in the arm shown below.
- Magnesium
the ion of magnesium it also has great biological importance, as it is present in 70% of our bones, favoring the action of enzymes, muscles and motor coordination. Its lack in the body can cause disorientation, hypertension, tremors, and a weak pulse. Therefore, we should eat the following foods that contain this element: nuts, grains and green vegetables. Also, excess magnesium in the body can cause drowsiness.
Magnesium also exists in seawater and minerals. It is used in metallic alloys, such as sacrificial metals (which oxidize in place of steel, preventing its corrosion), in “magnesium” wheels and in fireworks (offering the colors silver and white). A very important compound of magnesium is magnesium hydroxide (Mg(OH)2), which, when dispersed in water, at a concentration of approximately 7%, gives rise to a known suspension popularly as milk of magnesia, which serves as an antacid, fighting stomach acidity (heartburn) and as laxative.

By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/metais-alcalinoterrosos.htm