Ionization energy is a periodic property that indicates the energy needed to transfer the electron from an atom in a ground state.
An atom is in its ground state when its number of protons is equal to its number of electrons.
The transfer of electron(s) from the atom is called ionization. Therefore, the energy needed for it to happen is called ionization energy, also known as Ionization Potential.
The first electron removed is the one that is farthest from the nucleus of the atom. The distance facilitates the transfer because the farther away from the nucleus, which is positive, the less energy it takes for the electron to be taken out of it.
The next electron(s) need more energy. Thus, we can say that the 1st ionization energy (E.I) is less than the 2nd ionization energy. The 2nd, in turn, is smaller than the 3rd ionization energy and so on:
1st E.I
This is because the atomic ray it increases in size as each electron is removed from the atom. As a result, electrons are getting closer and closer to the atomic nucleus.
Check the successive oxygen ionization energies:
O -> O+: 1313.9 kJ mol-1
O+1 -> O+2: 3388.2 kJ mol-1
O+2 -> O+3: 5300.3 kJ mol-1
O+3 -> O+4: 7469.1 kJ mol-1
O+4 -> O+5: 10989.3 kJ mol-1
When, after removing an electron, the atom has more protons than electrons, that atom becomes a cation.
Read too:
- Ion, Cation and Anion
- ionization
This is what happens, for example, when we remove an electron from hydrogen. Hydrogen is made up of 1 proton and 1 electron.
After removing the electron, the hydrogen has only one proton in its nucleus. It means that the hydrogen was ionized and that it became a cation, which is the same as saying that it became a positive ion.
Ionization Energy in the Periodic Table
The atomic radius increases right to left and top to bottom on the periodic table.
Knowing this, the ionization energy increases in the opposite direction, that is, it is greater from left to right and from bottom to top.
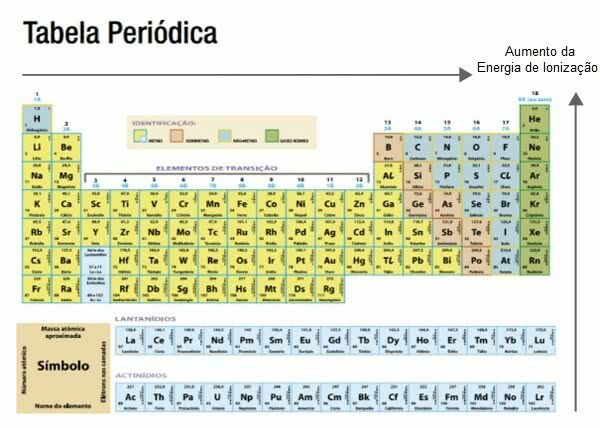
Among the elements that need less ionization energy are the alkali metals, for example, potassium.
Noble gases, in general, are those that require a higher ionization energy, for example, argon.
Removal Energy x Ionization Energy
Removal energy is very similar to ionization energy. The difference between the two is that the removal energy can be associated with photoelectric effects.
Photoelectric effects are electrons usually emitted by metallic materials exposed to light.
As a result, in the removal energy the removal of electrons does not follow a sequence as happens with the ionization energy.
In ionization energy, the first electrons removed are the most distant from the nucleus.
Electronic Affinity
THE electronic affinity it also influences the behavior of atoms, but in reverse.
This is the periodic property that indicates the energy released when an atom receives an electron. On the other hand, ionization energy is the energy needed to remove an electron from an atom.
Read too electropositivity and electronegativity.
Exercises
1. (PUCRS) Considering the position of the elements in the periodic table, it is correct to state that, among the elements listed below, the one with the smallest radius and highest ionization energy is the
a) aluminum
b) argon
c) phosphorus
d) sodium
e) rubidium
b) argon
2. (UEL) In periodic classification, the ionization energy of chemical elements INCREASES
a) from the ends to the center, in periods.
b) from the ends to the center, in families.
c) from right to left, in periods.
d) from top to bottom, in families.
e) from bottom to top, in families.
e) from bottom to top, in families.
3. (Uece) Let the following neutral atoms be represented by the hypothetical symbols X, Y, Z and T and their respective electronic configurations:
X → 1s2
Y → 1s2 2s2
Z → 1s2 2s2 2p6 3s2 3p6
T → 1s2 2s2 2p6 3s2 3p6 4s2
The one with the highest ionization energy is:
a) Y
b) Z
c) T
d) X
d) X
4. (Ufes) The first ionization energy of bromine (Z=35) is 1,139.9kJ/mol. Check the alternative that contains the first ionization energies of fluorine (Z=9) and chlorine (Z=17), respectively, in kJ/mol.
a) 930.0 and 1,008.4
b) 1,008.4 and 930.0
c) 1,251.1 and 1,681.0
d) 1,681.0 and 1,251.1
e) 1,251.0 and 930.0
d) 1,681.0 and 1,251.1
Check entrance exam questions with a commented resolution in: Exercises on the Periodic Table.