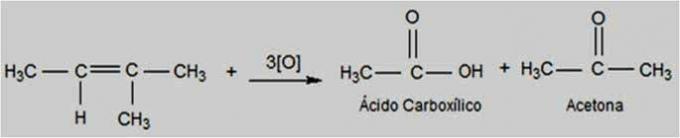
Aspirin®, which occupies the 3rd position in the ranking of the most consumed analgesics in the world, had its history started in 400 BC. Ç. The Greek Hippocrates used to prepare teas from the leaves and bark of the willow tree to relieve fever and headaches. Little did he know that this plant had salicylic acid in its composition, the compound that would give rise to Aspirin®.
In the year 1853, acid was first synthesized through the hands of French chemist Charles Gerhardt, he added acetate to the composition and obtained acetylsalicylic acid, but its formula was not used in the production of medicine.
Only in 1897, the chemist Félix Hoffman, who worked for the German company Friedrich Bayer & CO, developed the analgesic; which arrived in Brazil in 1901 and in 1906 the drug was internationally registered by Bayer.
Do not stop now... There's more after the advertising ;)
In 1969 the success of Aspirin® was proven in a space mission, Apollo 11, the drug was taken in the space capsules of astronauts.
Years passed and in 2002 studies indicated Aspirin® as an important factor in fighting cancer, heart attack and arthritis, and that it can still prevent Alzheimer's disease. Already in the year 2010 new studies showed more properties of the product, it can reduce the risk of death in victims of breast cancer.
When it comes to analgesic and antipyretic action, aspirin is second only to Paracetamol and Ibuprofen.
It is used daily by approximately 216 million people worldwide.
By Líria Alves
Graduated in Chemistry
Brazil School Team
Chemistry Curiosities - Chemistry - Brazil School
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "History of Aspirin®"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/historia-aspirina.htm. Accessed on June 28, 2021.