Free radicals are organic substituent groups that have a free, ie unshared or unpaired electron. These radicals are very unstable and extremely reactive, easily attaching to other positively charged species that are nearby.
Obtaining free radicals occurs through a break or homolytic split, also called homolysis of covalent bonds. This word, "homolysis", indicates that there is a equal disruption of the two electrons in the bond, being that each atom gets one of the electrons they were sharing before; for, in Greek, homo means equal and lysis it is break, that is, an equal break of the link. See an example below:
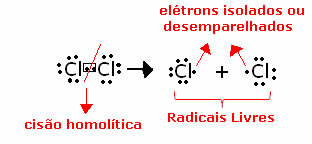
Our body produces free radicals in breathing and burning cells. They are produced in our bodies because in certain cases they are beneficial. For example, they are used as a means of defense, attacking foreign bodies that manage to enter our bodies.
However, these radicals are highly toxic chemical species, which attack the cells of our body and can lead to their death. This aggression causes, for example, a
acceleration in aging, since radicals bind to other species in our body, thus damaging healthy cells and producing toxic and undesirable compounds. Some species that can be destabilized are proteins, DNA and sugars.A good diet helps to reduce and control the release of these radicals. This food must be rich mainly in antioxidants, which are able to react with free radicals and neutralize them.
However, the opposite is also true, poor diet, associated with other risk factors, increases the production of free radicals and the degradation of our body. See below for the main factors that increase the production of free radicals and consequently cause premature aging:

By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Source: Brazil School - https://brasilescola.uol.com.br/quimica/radicais-livres.htm


