Have you ever noticed that shortly after we season the salad, the vegetables start to wither? This happens because of a very common process called osmosis.
for us to understand what is osmosis, first we must know some important concepts, such as solute and solvent. This last term refers to a substance capable of dissolving others, such as water. The solute is the substance that is dissolved by a solvent, such as salt.
When there is a lot of solute in a solution, we say that the medium is hypertonic; and when the solute is found in a small amount, we say that the medium is hypotonic. the middle is isotonic when the solute and solvent concentration are the same.
THE osmosis is a very common process between cells and is characterized by the movement of water through the membrane from a less concentrated (hypotonic) medium to a more concentrated (hypertonic) medium. The solvent crosses the cell membrane and migrates from a region where it is found in greater amounts to a place where it is present in lesser amounts.
Imagine the salad example again. In the presence of salt, the external environment has a greater amount of solute, that is, it becomes hypertonic. The water then leaves the plant cells by osmosis in an attempt to leave the internal and external environment with the same amount of solute and solvent.
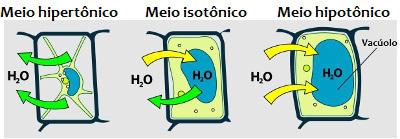
Analyze the movement of water into or out of the cell in different media *
Now imagine another situation: you put a cell plant in a hypotonic medium and shortly thereafter notice that the cell has increased in size. What happened in this case?That's right! Water migrated from the external environment to the internal environment of the cell because the intracellular environment is hypertonic. If that same cell were placed in a hypertonic medium, the cell would lose water, so it would wither.
Now you know why the salad wilts when salt is added: she is losing water through osmosis! Therefore, avoid putting the seasoning too long before serving, as that way you serve a much more beautiful and tasty product.
*Image Credit: Wikimedia Commons
By Ma. Vanessa dos Santos



