At molecules they are structures that have a determined molar mass and a relatively small and exact number of atoms bonded to each other covalently, that is, through the sharing of electrons.
See three examples:
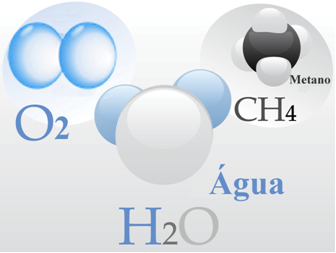
- Oxygen gas: Its molecules are each formed by a double bond, that is, by the sharing of two pairs of electrons between two oxygen atoms (O2);
- Water: it is formed by H molecules2O. Which means that each molecule has two hydrogen atoms sharing a pair of electrons each with an oxygen atom.
- Methane: this compound is formed by CH molecules4, in which four hydrogen atoms covalently bond to one carbon atom.
Molecular substances have some characteristic properties, such as:
- Varied solubility in water and other solvents;
- They can present themselves in the three physical states;
- Generally speaking, they are electrical insulators, both solid and liquid.
All molecules are formed by non-metallic elements, that is, they only include hydrogen, non-metals and semi-metals. But these elements can also form substances that are only present in a solid state and that have properties very different from those of molecules. these are the
macromolecules.Macromolecules, also known as covalent solids or covalent network solids,they are structures with a very high and imprecise molar mass, in addition to being formed by a large and indeterminate amount of atoms that covalently bond to form three-dimensional lattices. The macromolecules form the covalent crystals or atomic crystals.
For example, carbon atoms can share electrons in many ways and form many different simple substances. This property that the same chemical element has to form two or more different simple substances is given the name of allotropy.
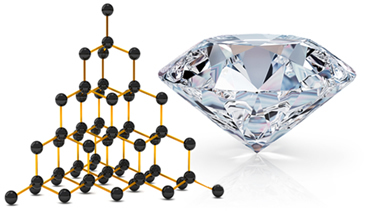
Two allotropic varieties of carbon that form macromolecules are the Diamond and the graphite. In the images below you can see that, in the case of diamond, each carbon atom binds to four other carbon atoms and gives rise to a macromolecule with a tetrahedral structure.
Meanwhile, the graphite structure is formed by hexagonal rings contained in the same plane. Carbon atoms make one double and two single bonds.

But macromolecules are not just made up of a single type of element; they can also be formed from atoms of different chemical elements.
An example is silicon dioxide (quartz), whose macromolecules have each silicon atom surrounded by four carbon atoms and each oxygen atom linked to two silicon atoms.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/moleculas-macromoleculas.htm

