O Petroleum it is a homogeneous mixture extremely important for several branches of activities developed by man. However, instead of oil itself, man uses the substances present in this compound, such as:
Gasoline
Diesel oil
Lubricant
Kerosene
asphalt mass
Paraffin
Natural gas
In order for all these substances to be used, they must go through a process of separation of mixturescalled fractional distillation. Just separated, each of the components can be used for the most diverse branches of activities.
Now, you know what a fractional distillation is.? In this text you will have access to all information about the method that separates the petroleum components.
What is Fractional Distillation?
Fractional distillation is the method used exclusively to separate substances present in a homogeneous mixture that must have at least two miscible liquids (liquid dissolved in liquid) and that do not have very close boiling points each other.
Equipment used in Fractional Distillation
Heat source (heating plate or bunsen burner);

Representation of a Bunsen burner
Flat-bottomed flask (receives the homogeneous mixture that will be separated);
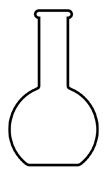
Representation of a flat-bottomed balloon
Universal support (for grip support);
Grips (used to secure the fractionation column);

Representation of the universal support with attached claw
Fraction tower or column;

Representation of a Fraction Column
Wooden stopper (it is positioned above the fractionation column to prevent steam from escaping and not entering the condenser);

Representation of a wooden stopper
Thermometer (used to control the temperature of the experiment);

Representation of a Thermometer
Condenser (condenses the material vapor with a lower boiling point);

Representation of a Condenser
Erlenmeyer or beaker (collects the condensed liquid);
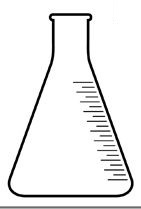
Representation of an Erlenmeyer
To make it easier to understand how fractional distillation is carried out, we will use as an example the mixture formed by water (whose boiling point is 100 OC) and acetone (whose boiling point is 58 OÇ). See below a representation of the assembled fractional distillation equipment and the step-by-step of how the process takes place:

Equipment set for fractional distillation
Initially, the mixture of water and acetone is added to the flat-bottomed flask and then started to be heated by the heat source (bunsen burner or heating plate);
When heated, both water and acetone are turned into steam. Thus, inside the balloon, we have water vapor and acetone. Both tend to go to the top exit of the balloon;
Right after the upper outlet of the flat-bottomed balloon, the fractionation column is connected. This column has several glass or porcelain balls inside, becoming an obstacle for the vapors that come out of the flat-bottomed balloon. The lighter or less dense vapor always passes through the fractionation column;
As the boiling point of acetone is lower than that of water, therefore, acetone vapor is less dense than water vapor. Thus, the vapor that will pass through the fractionation column will be that of acetone;
When passing through the fractionation column, the acetone vapor will necessarily enter the condenser, which is connected right after the end of the column. When entering the condenser, the acetone vapor undergoes the phenomenon of condensation and changes from the vapor state to the liquid state.
In this way, in the Erlenmeyer flask, we will have the liquid acetone separated from the water, which will be retained in the flat-bottomed flask.
By Me. Diogo Lopes Dias


