Gallium is a chemical element with an atomic number (Z) equal to 31 and its symbol is Ga. It belongs to family 13 (or group IIIA, according to the old numbering), which is the family of boron, being a “silver” colored metal similar to aluminum.
One of its most interesting properties is its melting point, which is low compared to almost all metals (except mercury) previously known, approximately 29.76°C. Thus, under ambient conditions, it is usually solid state. However, on warmer days, it melts into a liquid state. That's why, if we hold this metal in our hands, it will start to melt, because our temperature is higher than its melting point.

When storing gallium, it cannot fill the entire container as it expands as it solidifies
There are many videos on the internet that show a spoon that melts when placed in a glass of water. In fact, these spoons are made from gallium, not other more common metals or metal alloys such as aluminum or steel. steel. Thus, when the gallium spoon is placed in warm water, it becomes liquid. See more about this in the text “
Proposal for an experimental class on melting point”.Gallium also has another different feature, which is the huge range between melting and boiling temperatures. As already mentioned, its melting point is around 29.76°C, but its boiling point is around 2204°C.
Gallium was discovered between 3 am and 4 am on the 27th of August 1875 by the French chemist Paul Lecoq de Boisbaudran. An interesting aspect is that years earlier, in 1868, Russian chemist Dimitri Ivanovitch Mendeleyev (1834-1907) proposed the Periodic Table, but left a gap for an element that until then was unknown. Mendeleyev called it eka-aluminum, because he spectacularly predicted that in the horizontal row of boron, between aluminum and uranium, this element would lie.

French chemist Paul Lecoq de Boisbaudran – discoverer of gallium
Mendeleyev even predicted the properties of this element, such as its atomic weight, which would be 68, and its specific gravity, which would be 5.9. Thus, Lecoq discovered an element with an atomic weight of 69 and a specific gravity of 4.7, indicating that Mendeleyev was wrong. However, Mendeleyev said that Lecoq's sample was not pure enough and that he should repeat the experiments.
That's what Lecoq did, and surprisingly Mendeleyev was right, the specific gravity of this new element was 5.9. So it really was the eka-aluminum that Mendeleyev had envisioned.
Lecoq gave the name “Gallium” to the discovered element in reference to the Latin name for France, which is Gallia. But there are some who claim that, in fact, his aim was another, because in French Le coq means “the cock” and in Latin it is gallus.
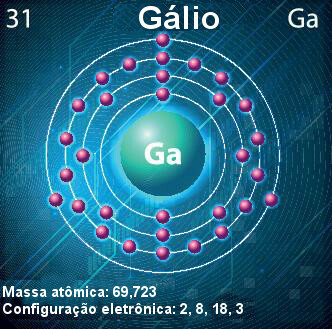
Gallium atom — symbol, atomic number, atomic mass and electron configuration
Another property of gallium is that it corrodes other metals. On the internet there are some videos that show the placement of a little liquid gallium on top of an aluminum can. After a few hours, it is possible to break it with your hands very easily.
Among the applications of gallium, we can highlight:
* It is used in the manufacture of mirrors;
* It is a semiconductor and conducts heat twice as much as the iron. Therefore, it is used in the production of diodes, LEDs, transistors and temperature, light and magnetic field sensors;
* In thermometers used for very high temperatures;
* In the manufacture of metal alloys that need to have low melting points;
* Obtaining hydrogen gas through contact between the aluminum gallium alloy and water;
* The Ga-37 isotope is radioactive and is used as a tracer in tests to detect diseases and tumors.
By Jennifer Fogaça
Graduated in Chemistry
