Density is a physical quantity that measures the concentration of matter of a body in a given volume.
It is obtained by the ratio between body mass and volume and is measured in kg/m3 in the International System of Units (SI).
The density of a body is inversely proportional to its volume, that is, the greater the volume, the lower the density. Note this relationship in the density formula:

Density is the property that determines whether a body will float or sink in a liquid. If the material is less dense than the liquid, it will float, but if it has a higher density than the liquid, it will sink.
All materials found in nature have a density that can be measured. Density depends on the type of material, of physical state where he finds himself and the conditions of temperature and environmental pressure.
How to calculate density?
To calculate the density of a substance or a mixture of substances, divide the body's mass by its volume, according to the formula:
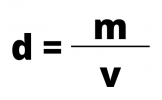
Where:
- d = density measured in kg/m3, g/cm3 or g/ml
- m = mass measured in kg or g
- v = volume measured in m3, cm3 or mL
It is important to keep in mind that as the volume of a body changes under different conditions of temperature and pressure, the density will be different when the temperature and pressure change.
For example, water has a density of approximately 1 g/cm3 in ambient condition, but when it becomes ice its density decreases to 0.92 g/cm3 - it is because of this difference in density that ice floats on water.
 Ice floats on water because its density is lower in a solid state than in a liquid state.
Ice floats on water because its density is lower in a solid state than in a liquid state.
Practical examples of density
To understand in practice what density is, imagine two containers, one with 1 kg of lead and the other with 1 kg of cotton. Both have the same mass, ie 1 kilo.
However, as cotton is much lighter than lead, a much larger amount of this material is needed for the masses of both containers to equalize.
Thus, the space occupied by cotton will be much larger than the space occupied by lead. This means that lead has a much greater amount of concentrated matter and therefore a higher density than cotton.
Now see other examples of practical situations that are explained by the difference in density of materials:
Ice cubes
When placed in a glass of water, ice cubes float because their density (0.92 g/cm)3) is less than the density of water (1g/cm3).
When ice cubes are placed in a glass of alcohol, the ice cubes will sink because their density (0.92 g/cm)3) is greater than the density of ethanol (0.79 g/cm3).
 Ice sinks in a glass of alcoholic beverage because the density of alcohol is less than that of water.
Ice sinks in a glass of alcoholic beverage because the density of alcohol is less than that of water.
Styrofoam and nail
Another example that helps to understand density is to observe the behavior of a Styrofoam board and a nail made of steel when placed in water.
The nail immediately sinks because its density (0.78 g/m)3) is much greater than the density of water. This means that steel has a large amount of matter concentrated in a small volume.
On the other hand, when the styrofoam board is placed in water it floats because the density of the styrofoam is less than the density of water. Unlike steel, Styrofoam is a substance that has little concentrated matter.
 The density of Styrofoam is less than that of water, so the board floats.
The density of Styrofoam is less than that of water, so the board floats.
Specific mass and density
Density is also used to refer to density, but they cannot always be understood as synonyms.
THE density refers to a body, which can be composed of just one substance or a mixture of substances, such as a solution of water with sugar.
THE Especific mass, in turn, refers to the density of a homogeneous substance specific, such as aluminum, lead or water.
This means that when a body is composed of just one substance, its density is given by its specific mass. However, when the body is heterogeneous, it will be necessary to calculate its density as the ratio of mass to volume.
Substance density
See the density (or specific mass) of some substances in the table below:
| Substance | Density |
|---|---|
| Water | 1,0 |
| Air | 1,2 |
| Steel | 7,8 |
| Aluminum | 2,7 |
| Lead | 11,3 |
| Copper | 8,9 |
| Ethanol | 0,79 |
| Iron | 7,86 |
| Ice | 0,92 |
| Milk | 1,03 |
| wood | 0,5 |
| Gold | 19,3 |
Absolute density and relative density
Absolute density refers to the density of a given body or substance. Relative density, in turn, is the density of one material compared to another.
The calculation of the relative density is the ratio between the two densities, which are calculated by dividing the mass by the volume. The density formula can be expressed by:
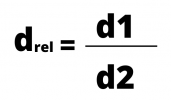
or
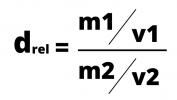
Relative density is often used to compare the density of substances in relation to water, whose density is 1g/cm3.
Density unit of measure
The unit of measure used in the International System (SI) is kg/m3, but it is common to find this magnitude also expressed in g/cm3 and g/ml. The relationship between these units of measure is:
1 g/cm3 = 1 g/ml = 1000 kg/m3
See also the meaning of chemistry and volume measurements.
