THE Water can be found in planet Earth in three different physical states: solid, liquid and gas.
The conditions that determine these states are the temperature and the atmospheric pressure where the water is located.
The surface of our planet has 70% water in its composition, most of it is in a liquid state and can be found in rivers, lakes, lakes, seas and oceans.
At the poles of the planet, water is in a solid state, forming the large glaciers and the atmosphere it is composed of many gases, including water vapor.
Index
- Physical states of water
- Physical state changes
- Vaporization
- Solidification
- Condensation
- Fusion
- Sublimation
Physical states of water
Check out the physical states of water and its definitions:
- Liquid state: it does not have a defined shape and is suitable for the place or container in which it is found.
- Solid state: it has a rigid and regular structure, which does not adapt to the places where it is inserted, it usually takes the form that liquid water has before it solidifies.
- Gaseous state: it does not have its own shape, the molecules are very small and cannot be seen at naked eye, but they are present everywhere and are extremely important for the survival of beings alive.
Physical state changes
At physical state changes occur when changes in temperature and/or atmospheric pressure occur.
These changes in the physical state of the water are responsible for the formation of the water cycle, which allows the maintenance of the atmosphere that guarantees terrestrial life.
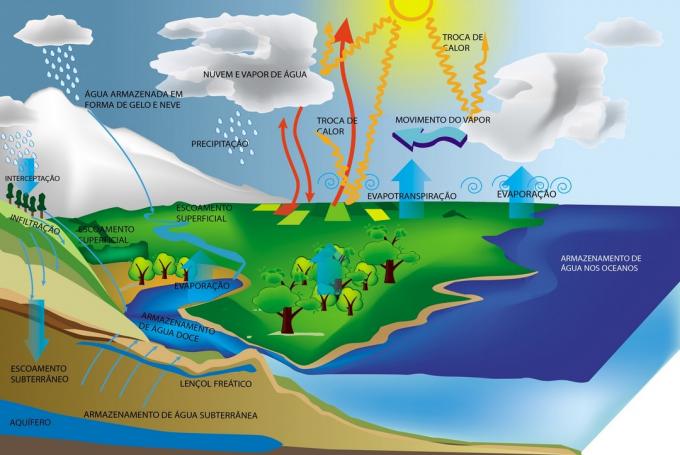
There are five possibilities for changes in the physical state of water, they are: vaporization, solidification, condensation, fusion, sublimation. Let's meet them?
Vaporization
Vaporization is the transformation of water from a liquid to a gaseous state. It can occur in three different ways:
- Boiling: The boiling point is the temperature at which water begins to boil and turn into steam. When you are at sea level, that temperature is 100 °C.
- Evaporation: occurs slowly and gradually, but increasing temperature can accelerate the process. This is what happens when we put clothes to dry on the clothesline or when rainwater falls on the ground.
- Heating: This is the fastest form of vaporization, as water evaporates at temperatures above the boiling point. This is what happens when a pan is too hot and just a drop of water falls into it, instantly turning it into steam.

Solidification
- Free Online Inclusive Education Course
- Free Online Toy Library and Learning Course
- Free Online Preschool Math Games Course
- Free Online Pedagogical Cultural Workshops Course
THE solidification occurs when water changes from a liquid to a solid state, this occurs when it reaches 0 °C.
This process is responsible for the formation and maintenance of glaciers, snow, and ice that we consume in beverages.

Condensation
THE condensation it is the process in which water vapor is transformed into liquid water, that is, it is the passage from the gaseous state to the liquid. It can also be called liquefaction.
For condensation to occur, it is necessary that the water vapor comes into contact with lower temperatures.
Condensation is the process responsible for the precipitation of rainwater, as it is through this process that the water vapor contained in the atmosphere returns to a liquid state.
The water droplets that form on the lid of the pan when we are cooking or the droplets that form accumulate on the outside of a glass that contains a cold beverage are also formed by the process of condensation.

Fusion
THE Fusion is the transformation of the solid state into a liquid state and happens when the state temperature solid that is below 0 °C starts to increase and reaches 0 °C, that is, the melting point of water is 0°C.
It is important to remember that the temperature remains constant at 0 °C until melting is complete in all the solid state water.

Sublimation
At sublimation the transformation of water from a gaseous state to a solid occurs without going through the liquid state, that is, directly, skipping a physical state.
This transformation occurs in sudden changes in temperature, but it is uncommon in everyday life, they happen more in the industrial environment, dry ice is an example of sublimation of water.

See too:
- water properties
- Water desalination
- Tocantins-Araguaia Basin: Main rivers, location, hydroelectric plants and importance
- Paraguay River – Map, source, tributaries, extension, commercial activities
The password has been sent to your email.

