Chargeelectric is a property of matter, just like the pasta. The macroscopic electrical charge of a body arises from the difference between the number of protons and electrons, in this case we say that the body is charged or electrified.
On the other hand, when the amount of electrons and protons is the same, we say that the body is neutral. Therefore, even when neutral, the bodies still have electrical charges, however, these are balanced.
The electrical charge originates from subatomic particles: protons have the smallest positive charge, while electrons have the smallest negative charge. You neutrons, in turn, are electrically neutral particles.
Lookalso: Basics of Electrostatics
Types of electrical charge
There are two types of electrical charge, the positive and the negative. Both are defined exclusively by algebraic signs, by convention the charge on the electron is assigned the negative sign and the charge on the proton the positive sign.
These signs, however, are merely arbitrary. For example, there would be no problem if, at some point, the charge on the electrons were to be described with the positive sign and the charge on the protons became negative. The important thing is that these two charges
attract when your sign is differentotherwise they tend to repel each other.Mind Map: Electric Charge

*To download the mind map in PDF, Click here!
electric charge unit
The unit of measurement of electrical charge is the Coulomb (C), as a form of homage to the French physicist Charles Augustin de Coulomb (1736-1806), responsible for determining the mathematical law that describes the attraction and repulsion force between charges. However, the Coulomb quantity is not listed as one of the fundamental quantities of the international system of units.
In fact, it is a greatness derived from Ampereand is used for electric current measurements. The Coulomb of electrical charge is equivalent to the amount of charge that is carried by an electrical current of 1 amp during the 1 second time interval. Therefore, we say that 1.0 C is equivalent to 1.0 A.
Lookalso: Coulomb's Law
Quantization
The electric charge is quantized, that is, the load modulus of a body is determined by a integer multiple of an amount of cargo: a chargefundamental. The fundamental charge (and) is the smallest electrical charge that exists in nature, it is the charge that can be found in protons and electrons, its modulus is approximately 1,6.10-19 Ç.
how to calculate
Based on the fundamental load modulus, it is possible to find out what the missing or excess number of electrons for a body to present a certain electrical charge, note:
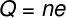
Q — total electrical charge (C)
no — number of missing or excess electrons
and — fundamental electrical charge (1.6.10-19 Ç)
Using the quantization of electrical charges, represented by previous formula, we can calculate what must be the amount of electrons, missing or excess, needed to produce a total electric charge of 1.0 C in a body:
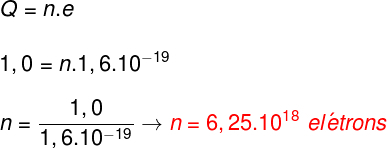
The last result shows that, for a body to be loaded with 1.0 C, it is necessary that they be removed 6.25.1018 electrons of its atoms. Therefore, it is easy to see that 1.0 C of electrical charge is a huge amount of that charge.
Due to the forces of attraction and repulsion between electrical charges, it is natural that all bodies seek the state of electrification with the lowest possible energy, that is, the most bodies that are around us is electrically neutral. For a body to become electrically charged, it must receive or donate electrons to its surroundings.
Furthermore, it is not possible to electrify a body by ripping it out or supplying it with protons, since these particles are about 1840 times more massive than electrons, in addition to being strongly bonded to other protons in the coreatomic. Therefore, for a body to receive or donate electrons, it must suffer at least one of the three electrification processes: friction, contact or induction.
Lookalso: what is electric field?
How to know the electric charge of an atom
Atoms are generally neutral, so their total electrical charge is null. However, if the atoms are ionized, and have their electrons ripped off, they will present a positive electric charge, due to their lack of electrons or the excess of protons.
Positively charged atoms are called cations, while atoms that receive electrons, and become negative, are called anions. We can determine the electrical charge of the atomic nucleus or the electrosphere through your atomic number Z.
In the following example, we calculate the electrical charge of a helium nucleus (α particle, Z = 2), which has two protons and two neutrons:

solved exercises
Question 1) During an electrification process, a body receives an amount of 2.0.1015 electrons, becoming electrically charged, with an electrical charge of:
a) 3.2.10-4 Ç
b) 1.6.10-18 Ç
c) 3.2.10-5 Ç
d) 0.32.10-5 Ç
e) 320.10-1 Ç
Template: Letter a
Resolution:
Let's use the electric charge quantization formula, note:
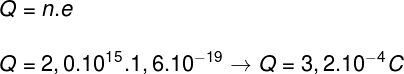
After replacing the values provided by the statement in the formula, we find that the electrical charge of the body after electrification will be 3.2.10-4 Ç. Therefore, the correct alternative is the letter A.
Question 2) A body has 1.2.103 electrons less than protons. Determine the sign and magnitude of the electrical charge of this body.
a) Negative, 0.92.10-13 Ç
b) Positive, 1.92.10-13 Ç
c) Negative, 1.92.10-16 Ç
d) Positive, 1.92.10-16 Ç
e) Negative, 1.6.10-14 Ç
Template: Letter D
Resolution:
To calculate the electrical charge of this body, just take into account the difference between the number of protons and electrons, observe:

As explained in the statement, the body has more protons than electrons, so its charge will be positive.
Question 3) Determine how many electrons need to be removed from a body for its electrical charge to be 6.4 C.
a) 4.0.1015 electrons
b) 4.0.1019 electrons
c) 2.5.1018 electrons
d) 3.5.1021 electrons
e) 1.6.1012 electrons
Template: Letter B
Resolution:
The exercise asks us to find the number of electrons, so we will do the following calculation:
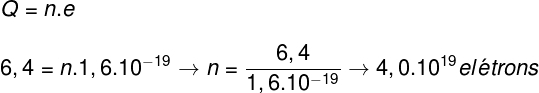
Based on the resolution of the exercise, we find that it is necessary to remove 4.0.1019 electrons of a body, so that its electrical charge is 6.4 C.
By Me. Rafael Helerbrock
