As explained in the text “Types of Intermolecular Forces”, the molecules of substances in the three physical states (solid, liquid and gas) are attracted by one of the intermolecular forces.
The three known intermolecular forces are: induced dipole - induced dipole, permanent dipole - permanent dipole and hydrogen bonding. Among them, the hydrogen bond is the strongest. Some authors used to refer to this intermolecular force as hydrogen bonds; however, the correct term accepted by IUPAC is “hydrogen bonding”.
This type of interaction occurs when the molecule has hydrogen bonded to fluorine, nitrogen or oxygen, which are strongly electronegative atoms.

The hydrogen bond is an extreme example of the permanent dipole-permanent dipole bond. For the hydrogen of a molecule constitutes a positive pole, which binds to one of those fluorine, oxygen or nitrogen atoms of another molecule, which constitute their negative pole.
Normally, intermolecular bonds occur with substances in liquid and solid states. Also, since it is a very intense force of attraction, it takes a very high energy to break it.
A substance that has this intermolecular force is water itself. Note how this happens in the illustration below:
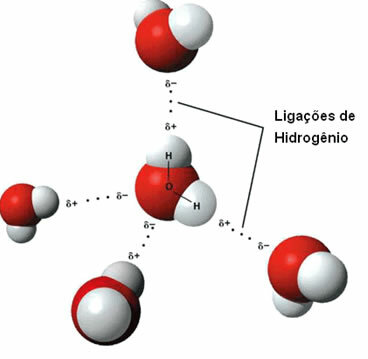
Note that each water molecule is spatially surrounded by four other water molecules, with the bonds of hydrogen occur by the bond between the hydrogen of one molecule (positive pole) with the oxygen of another (pole negative).
Hydrogen bonds explain various phenomena in nature, see the following examples:
- The fact that ice floats on water: Ice is less dense than water and consequently floats on it. This is because while in the liquid state the hydrogen bonds that occur between the water molecules are arranged in a disorganized form, the hydrogen bonds in the ice molecules are more spaced and organized, forming a rigid hexagonal structure, which makes the molecules occupy a much larger space than they would if they were in the state. liquid.

Do not stop now... There's more after the advertising ;)
This is even why if we put water in the full volume of a bottle and put it later in a cooler, its volume will expand and the bottle will crack.
Thus, there will be the same amount of molecules per unit of volume, which decreases the density, according to the density formula: d = m/v. There will be empty spaces between the formed hexagons, decreasing the density of this substance.
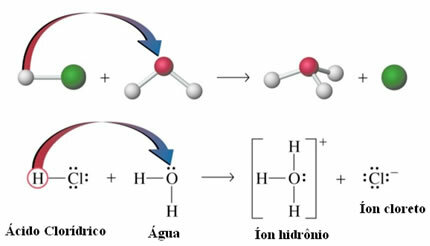
- Acid ionization: Although hydrogen bonds are approximately ten times weaker than covalent bonds; under certain circumstances they manage to break the covalent bonds. For example, in the case shown below, hydrochloric acid is dissolved in water. The oxygen in the water attracts the hydrogen bound to the acid's chlorine more than the chlorine itself, giving rise to hydronium ions (H3O+) and chloride (Cl-). This phenomenon is called ionization:
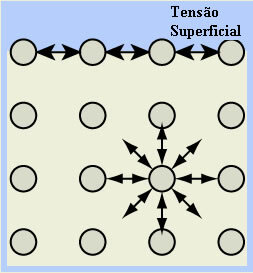
- Surface tension of water: the molecules on the surface of the liquid are attracted by hydrogen bonds only with the molecules on its side and below, as there are no molecules above. Molecules that are below the surface, on the other hand, carry out this type of bonding with molecules in all directions, the result is the formation of a kind of film or thin layer on the surface of the water, which involves.
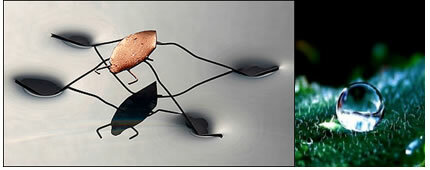
This explains the fact that insects can remain on it and also the phenomenon of the spherical shape of water droplets.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Hydrogen Bonds"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/ligacoes-hidrogenio.htm. Accessed on June 27, 2021.
Chemistry

Water pollution, physical aspects of water, chemical aspects of water, biological aspects of water, industrial waste, heavy metals, drinking water, organic matter, water turbidity, sewage.


