Acid rain it is a atmospheric phenomenon which occurs especially in countries with a high level of industrialization. It consists of precipitation with high acidity, that is, rain has a high concentration of acids such as sulfur dioxide. This phenomenon can cause serious environmental problems and also damage the health of living beings.
The term acid rain was first used by the English chemist and climatologist Robert Angus Smith in 1852. Smith used it to describe, through a study, the situation experienced in Manchester, UK, when precipitation with high acidity occurred in the period of Industrial Revolution.
Origin of acid rain
THE rain usually has a certain degree of acidity due to the presence of oxides in the air. Acidity can be measured using a numerical scale known as pH. On this scale, a pH 7 solution is considered neutral. The pH considered normal for rain is around 5.6. Therefore, the lower the pH, the more acidic the solution. To be considered acidic, rain must have a pH below 5.5.
readalso:types of rain
Do not stop now... There's more after the advertising ;)
THE acid rain can be of natural origin or anthropic. The main natural generators of acid rain are the volcanoes, which emit gases, particles, sulfur compounds and dust into the atmosphere; and the biological processes occurring in soils, swamps and oceans, in addition to animal and plant respiration.
The main anthropogenic contributors to acid rain are related to environments with large concentration of industries and vehicles. the burning of fossil fuels for energy generation and the gases emitted by vehicles favor the formation of acid rain.
also know: Acid Rain Chemistry
How is it formed?
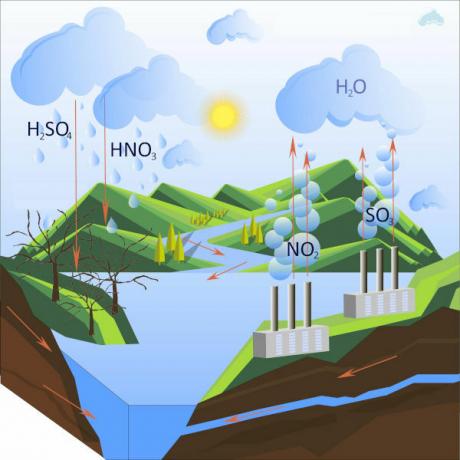
The formation of acid rain occurs through the reaction between oxides and water particles, which form acids.
Precipitation with high acidity occurs when there is a high concentration of gases such as sulfur dioxide and nitrogen in the atmosphere, which in contact with droplets of water suspended in the air, react to form acids that, when precipitating, give rise to acid rain.
It is worth saying that the acid rain does not only occur where there are gas emissions into the atmosphere. It is possible that these gases are transported by the wind to more distant regions, which may cause acid rain in other regions.
Mind Map: Acid Rain
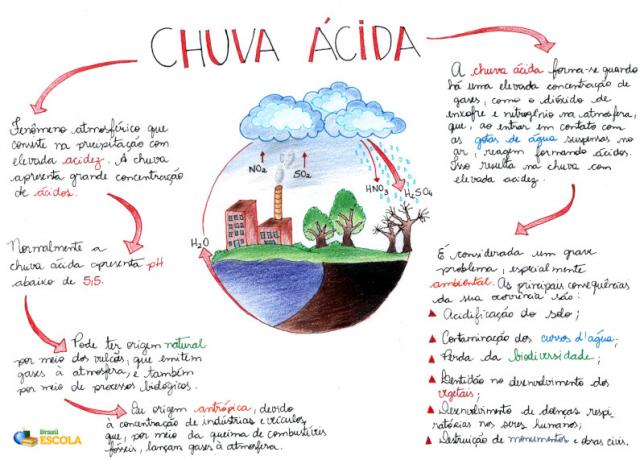
*To download the mind map in PDF, Click here!
Composition
The main gases that cause acid rain when they react with water particles suspended in the air are:
Sulfur dioxide: from the combustion of coal, manufacture of fertilizers and heating of sulphate group ores.
Nitrogen oxides: from the combustion of charcoal, the combustion of petroleum derivatives and cigarette smoke.
readmore:Oxides and acid rain
Main acids that make up acid rain:
Sulfuric acid |
Nitric acid |
Consequences

The destruction of vegetation is one of the main consequences of acid rain.
The occurrence of acid rain is considered a big problem, especially environmental, in different regions of the world. Since the period of the Industrial Revolution, with the increase of industries and produced goods, it has increased also atmospheric pollution, increasing the emission of polluting gases, favoring the incidence of this phenomenon.
The main consequences of acid rain are:
O ground can change when acid rain hits the surface, becoming acidic.
Soil contamination can also lead to contamination of water courses, such as rivers and lakes, as well as underground water reserves.
Contamination of watercourses can cause loss of biodiversity, with an increase in the concentration of acidity that impedes the development of aquatic life.
Vegetation is also harmed when excess acidity, upon reaching the vegetables, impairs their development, causing their growth to be slow.
Acidity causes the breaking of the surface of the leaves of trees, causing nutritional impoverishment.
Plants can become susceptible to pests and diseases.
Root growth slows down, impairing nutrient transport.
Depending on the acid concentration, the health of human beings can also be harmed. This is because the accumulation of sulfur dioxide in the body can lead to the development of respiratory illnesses.
It also causes havoc in cities, by corroding and destroying monuments and civil works.
Know more:Effects of acid rain on historic monuments
Acid rain in Brazil and worldwide
Acid rain in Brazil occurs mainly in the metropolises and several studies have been carried out in order to analyze this problem in the national territory. Because it often does not cause immediate effects, this phenomenon goes unnoticed by the majority of the population.
In the country, occurrences were recorded in the coal region of Santa Catarina, in the region of the industrial center of Sao Paulo it's from Minas Gerais and in the petrochemical complex region of the city of Camaçari, in the state of Bahia.|1| A well-known example in Brazil occurred in the Serra do Mar region in Cubatão, Sao Paulo, in 1977, due to the high emission of gases by industries installed in this area.
The concentration of gases sent to the atmosphere such as carbon monoxide, sulfur dioxide and benzene exceeded one thousand tons a day. Acid rain in São Paulo, according to data from the National Institute for Space Research (Inpe), has already reached a pH of 4.6 in the period between 1983 and 1985.
The main consequence was the damage caused to the Atlantic Forest, biome that covers this region. Much of the vegetation was lost due to acidity. Reforestation programs in the city of Cubatão have already been developed with the aim of reforesting the area and protecting the slopes that have lost their vegetation cover.
THE The region's population also suffered a lot. with the occurrence of rain, with high rates of respiratory diseases and children with malformation of the nervous system.
Worldwide, countries on the European continent and North America also suffer from the effects of acid rain.
See some countries that suffer from the high acidity of water:
U.S |
Approximately 10% of lakes in the Adirondack mountain region have a pH of less than 5. |
Germany |
More than half of the forests have suffered damage from acid rain. |
Poland |
Railroads have suffered severe corrosive effects. |
UK |
Acid precipitation caused damage to approximately 67% of forests. |
Source: The Global Ecology Handbook (1990).
Note
|1| The importance of studying acid rain in the context of the climatological approach. To access, Click here.
By Rafaela Sousa
Graduated in Geography
Would you like to reference this text in a school or academic work? Look:
SOUSA, Rafaela. "Acid rain"; Brazil School. Available in: https://brasilescola.uol.com.br/geografia/chuvaacida.htm. Accessed on June 27, 2021.
Chemistry

What are Artificial rains, cleaning the atmosphere, helping to extinguish fires, Chinese city Limfen, emission of sulfur and arsenic by thermoelectric plants, high toxicity, environmental organization Greenpeace, Olympic Games of Beijing.


