The analysis of the physical and chemical properties of compounds that carry out covalent bonds (by sharing electrons) shows us that there are great differences between these materials. But before we look at these characteristics themselves, let's see what the difference is between molecular and covalent substances.
At molecular substances they are those that are formed when atoms are linked through covalent bonds, giving rise to molecules of a determined number.
However, the covalent bond can also originate compounds in a network structure with a very large and indeterminate number of atoms, which are macromolecules. Such substances are called covalent compounds or covalent network solids. Some examples of these compounds are: diamond (C), graphite (C), silicon dioxide (SiO2) and Silicon carbide (SiC).
Now, let's look at its main properties:
- Physical state at room temperature: Under ambient conditions, molecular and covalent compounds are found in the three physical states (solid, liquid and gas).
Examples:
O Solid: sugar (sucrose), silica (sand), diamond, graphite;
O Liquid: water, acetone, ethanol;
O Gaseous: Hydrogen sulfide, chlorine gas, bromine gas, hydrogen gas.

- Melting and Boiling Point: In general, the melting and boiling points of these substances are smaller than those of ionic substances.
Covalent substances have higher boiling temperatures than molecular ones, always above 1000°C. This is because as its molecules are more closely joined, forming crystalline lattices, it is necessary to provide more energy to make them change their state.
Two factors interfere with the boiling and melting points of covalent and molecular compounds: a molar mass and the intermolecular force.
Do not stop now... There's more after the advertising ;)
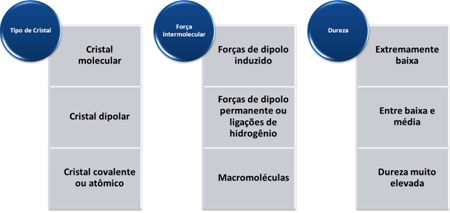
The greater the molar mass, the greater the inertia of the molecule and, consequently, the higher the boiling and melting point. If the molar masses are approximated, we look at the intermolecular forces. The most intense intermolecular force is that of hydrogen bonding, leading to a higher boiling and melting point. The intermediate is the permanent dipole and the weakest, which leads to a lower boiling and melting point, is the induced dipole.
- Electric current: In their pure form, both liquids and solids do not conduct an electrical current.
An exception is graphite, which conducts electrical current in solid form, because its double bond electrons resonate and therefore have a certain mobility.
- Solubility: Polars dissolve into polars and non-polars dissolve into non-polars.
- Tenacity: The resistance of covalent substances to impact or mechanical shock is low. In general, they are brittle solids, as shown in the case of glass, which is formed by sodium and calcium silicates.

- Toughness: In general, they have high hardness. With the exception of graphite, because its carbon atoms are linked to three other carbon atoms, forming hexagonal plates with certain mobility, making it soft. Because of this, it is even used as a lubricant.
The hardness of these substances varies according to the type of crystal, as shown in the table below:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Properties of Covalent and Molecular Compounds"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/propriedades-dos-compostos-covalentes-moleculares.htm. Accessed on June 28, 2021.
Ionic compounds, main characteristics of ionic compounds, bonding between ions, definitive transfer of electrons, electrostatic attraction forces between ions, negative and positive ions, anions, cations, ionic bonding, molecular structure he

