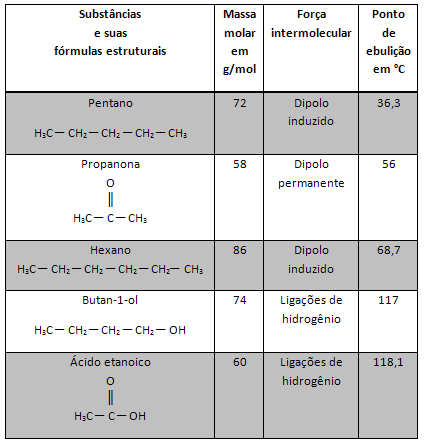
In the year 1911, the New Zealand scientist Ernest Rutherford presented to the scientific community its atomic model. Rutherford's model, also called the solar system model, was the third in the history of Atomistics (the first two being the model of Dalton and Thomson's model) and was considered the model that stimulated the entire evolution of knowledge about the constituent of matter, the atom.
The construction of the Rutherford model started from the study of the properties of X ray and radioactive emissions, culminating in the use of radiation on an inert artifact, that is, one that does not react easily.
Experiment carried out by Rutherford
The experiment carried out by Rutherford had the following apparatus and organization:
Component a - a sample of polonium (emitter of alpha radiation) placed on a lead block. In this block there was a small hole through which the radiation passed;
Component b: very thin gold blade positioned in front of the lead box;
Component c: Metal plate covered with fluorescent material (zinc sulfide) positioned behind, beside and a little ahead of the gold plate.
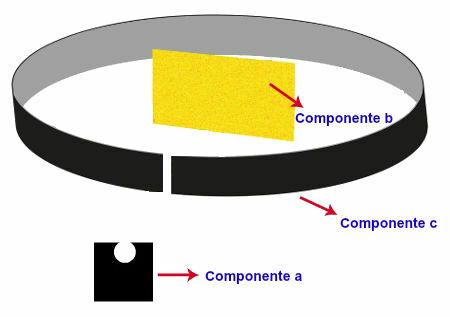
Representation of the experiment carried out by Rutherford
Rutherford Experiment Results
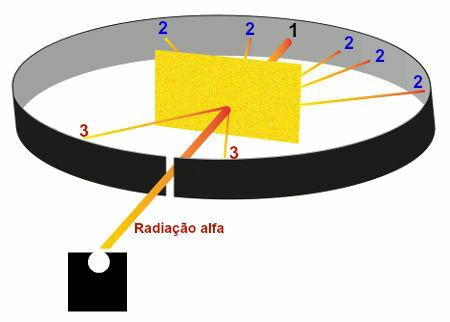
Representation of the results observed in the Rutherford experiment
Region 1: area that received a large part of the alpha radiation emitted by polonium, which showed that these radiations crossed the gold plate without suffering considerable deviations;
-
Region 2: several areas, located behind the gold plate, that received a small amount of alpha radiation but were not in the direction of the radiation exit hole in the lead box, which showed that these radiations suffered a large deviation after crossing the gold;
Do not stop now... There's more after the advertising ;)
Region 3: areas located in front of the gold plate that received an extremely small amount of alpha radiation, which showed that part of the alpha radiation collided with the plate and was bounced back.
Interpretations of Rutherford Experiment Results
Interpretation about region 1: Since most of the alpha radiation passed through the gold plate without any hindrance, this means that the atoms had large empty spaces (electrosphere), that is, regions that had nothing capable of influencing the radiation alpha;
Interpretation about region 2: The small amount of alpha radiation that underwent deviation passed close to a positive region (nucleus) of the atom, probably of small size, which promoted the deviation.
Interpretation about region 3: As an extremely small amount of alpha radiation was bounced off, this means that they collided with an extremely small region of the atom that had a positive characteristic.
Characteristics of the Rutherford Atomic Model
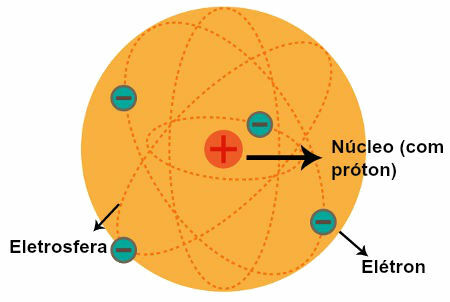
Representation of the Rutherford Atomic Model
After observations made by Rutherford, he formulated his atomic model, which had the following characteristics:
a) Nucleus (which has been compared to the sun in the solar system)
A central region of the atom that has:
positive particles (the protons);
low volume;
greater mass;
bigger density of the atom.
b) Electrospheres (which have been compared to orbits described by planets in the solar system)
Regions of the atom that have:
huge empty spaces between them;
particles of a negative nature (the electrons).
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Rutherford's Atomic Model"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/o-atomo-rutherford.htm. Accessed on June 27, 2021.
Chemistry

Matter, Dalton's atomic theory, substances, chemical element, John Dalton, atom, body, object, constitution of matter, rearrangement of atoms, sphere, mass.
Chemistry

Niels Bohr, Bohr's atom, atomic physics, stable atom, atomic model, planetary system, layers of the electrosphere, energy levels, electron shells, electron energy, Rutherford atomic model, excited state atom.