According to the Theory of Arrhenius, a base can be defined as any substance that in aqueous solution undergoes ionic dissociation, releasing the hydroxyl (OH) as the only type of anion-).
For a base to be considered strong or weak, we have to consider its degree of dissociation (α), which is given by:

If this degree of ionization is approximated to 100%,the base is considered strong.But if the value is equal to or less than 5%,the base is considered weak.
Examples of strong bases: Bases of alkali metals (LiOH, NaOH, KOH, RbOH, CsOH) and some alkaline earth metals (Ca(OH)2, sir (OH)2, Ba(OH)2). The Mg(OH)2 is an exception, being a weak base.
The dissociation degree of sodium hydroxide (NaOH) is equal to 95% at 18ºC, being an ionic compound by nature.
Examples of weak bases: O Ammonium hydroxide (NH4OH) and the bases of the others metals (from families 13, 14 and 15).
The degree of dissociation of ammonium hydroxide (NH4OH) is equal to 1.5% at 18°C, being a molecular compound in nature. Ammonium hydroxide is actually the ammonia or ammonia solution, often used to bleach hair. As it is an unstable base, NH
4OH decomposes under ambient conditions into water and ammonia gas (NH3(g)).Do not stop now... There's more after the advertising ;)

Typically, a base that is highly soluble in water also exhibits a high degree of ionic dissociation and is therefore considered a strong base. However, solubility in water should not be regarded as a reliable point to check the strength of the base. Pfor example, ammonia hydroxide is very soluble in water but is a weak base. Already the bases of alkaline earth metals, such as Ca (OH)2 (hydrated lime used in whitewash paints, like the one on the tree below), are considered strong, despite being poorly soluble in water.

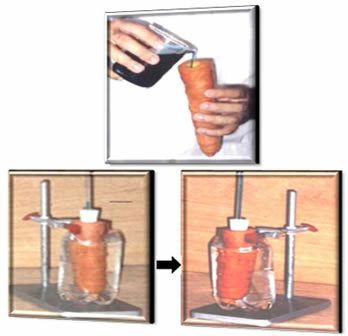
Since strong bases give rise to many ions in aqueous solution, they give rise to solutions that are good electrolytes, that is, they conduct electric current well. On the other hand, weak bases, although they also give rise to ionic solutions, conduct little electricity and are considered bad electrolytes.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "The strength or degree of dissociation from the bases"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/a-forca-ou-grau-dissociacao-das-bases.htm. Accessed on June 28, 2021.