Carboxylic acids are those organic compounds that have the carbonyl group attached to a hydroxyl group, that is, they have the carboxyl group below:

As these compounds were discovered, they were given names that were related to their origin or some of their characteristics, such as the following examples:
- H ─ COOH →Formic acid(recalling “ant”, as it was first obtained by distilling red ants);
- H3C - COOH → Acetic acid (from latin acetum, which comes from “sour = vinegar”, being the main component of vinegar);
- H3C - CH2 CH2 ─ COOH → Butyric acid(from English butter, which means “butter”; this acid contributes to the characteristic smell of rancid butter);
-
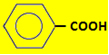 → Benzoic acid(in benzoin,an herb used in smoking).
→ Benzoic acid(in benzoin,an herb used in smoking). - HOOC - COOH →oxalic acid (from the greek oxys, which means “very acidic”).
However, over time, more and more carboxylic acids were discovered, in addition to thousands of other compounds organic and, as a result, it became very difficult to study all these compounds in an organized way and discuss research in several countries. Therefore, the IUPAC (International Union of Pure and Applied Chemistry, acronym that comes from English
International Union of Pure at Applied Chemistry) created rules for the nomenclature of all organic compounds according to the function to which they belong. These rules can be seen in the text. IUPAC Nomenclature.Do not stop now... There's more after the advertising ;)
In the case of carboxylic acids, the rule is basically as follows for those with a normal chain, that is, those that do not have branches:
ACID+ PREFIX + INFIX + HI CO
(indicates the (indicates the
the amount kind of
of carbons) Link)
Examples:
H ─ COOH →Acid metanHi co
H3C - COOH → Acid etanHi co
H3C - CH2 CH2 ─ COOH → Acid butanHi co
If there is more than one carboxyl group, it must be indicated by means of a prefix before the “oic” suffix, as in the examples below:
HOOC ─ COOH → Acid etanOdiHi co
HOOC - CH2 CH2 CH2 CH2 ─ COOH → Acid hexanOdiHi co
If there are unsaturations (double or triple bonds) and/or branches, it will be necessary to number the chain, starting from the carboxyl carbon. Remembering that if you have more than one branch, they must be written in alphabetical order, disregarding the prefixes di, tri, tetra, iso, sec, terc, neo etc.
Examples:
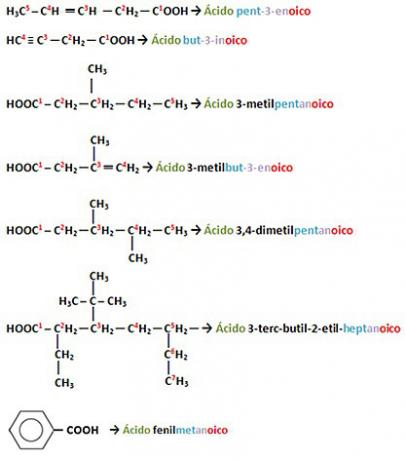
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Carboxylic Acid Nomenclature"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/nomenclatura-dos-Acidos-carboxilicos.htm. Accessed on June 28, 2021.
Alkanes nomenclature, hydrocarbon function, carbon valences, International Union of Pure and Applied Chemistry, IUPAC, saturated aliphatic hydrocarbons, single bonds, compounds Organic.