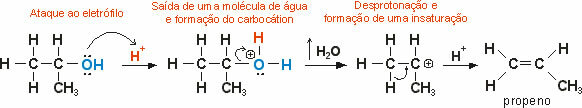
Nitration reactions are substitution reactions that occur through nitric acid (HNO3). This type of reaction occurs especially with alkanes and with benzene and its derivatives, where one of the hydrogen atoms attached to the chain or to the aromatic nucleus is replaced by the NO group.2, giving rise to a nitro compound and water.
See some examples:
1. Nitration of an alkane:
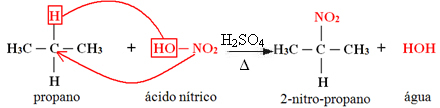
When the alkane has more than two carbons, a mixture of different substituted compounds is formed. The amount of each compound obtained will be proportional to the following order of ease with which hydrogen is released into the molecule:

In the case above, there is no tertiary carbon, there is only one secondary and two primary; thus, the largest amount of compound formed as a product will be 2-nitro-propane.
If there is enough nitric acid, another hydrogen can be replaced by the nitro group, giving rise to 2-dinitropropane (structure below), a compound widely used as an additive to diesel oil, increasing its octane rating and decreasing the emission of soot.
Do not stop now... There's more after the advertising ;)
AT THE2
│
H3C─C─CH3
│
AT THE2
2. Benzene nitration:
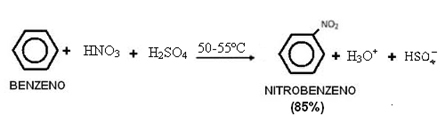
Note that this reaction occurs in the presence of heat, as the benzene is heated with a sulphonic mixture, ie, concentrated nitric acid with concentrated sulfuric acid. Sulfuric acid is a catalyst, causing the reaction rate to increase as benzene slowly reacts with nitric acid.
3. Nitration of benzene derivatives:
In such cases the place of substitution will depend on the substituent group or functional group attached to the aromatic nucleus. The texts "Steering Radicals in the Benzene Ring" and "Electronic effects of meta and ortho-to-directors radicals” explain more about how this happens.
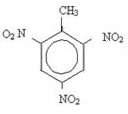
Aromatic rings can undergo nitration and give rise to explosives. An example is trinitrotoluene (2-methyl-1,3,5-trinitrobenzene), better known as TNT and there are several pigments used mainly in fabrics.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Organic Nitration Reactions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/reacoes-organicas-nitracao.htm. Accessed on June 28, 2021.