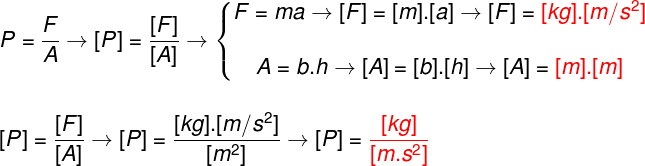Until 1824 it was believed that the thermal machines built could undergo a functioning perfect, that is, it was thought that they could reach 100% yield, or something close to that value. In other words, scientists at the time believed they could make use of all thermal energy supplied to these machines – that is, they believed they could transform all that energy into work.
Engineer Sadi Carnot was responsible, at the time, for making demonstrations in which it was impossible to obtain 100% yield. Sadi proposed that an ideal theoretical thermal machine would work through a particular cycle, now called Carnot Cycle.
In his demonstration, Carnot conceptualized two postulates, which were proposed even before the first law of thermodynamics was enunciated. See what Carnot's postulates enunciate:
1st Postulate of Carnot
- No machine operating between two fixed temperatures can yield greater than Carnot's ideal machine operating between those same temperatures.
2nd Postulate of Carnot
- When operating between two temperatures, the machine ideal of Carnot has the same performance, whatever the operating fluid, and is completely reversible, without adding energy.
According to the postulates enunciated by Carnot, we can see the guarantee that the efficiency of a thermal engine is a function of the temperatures of the hot and cold sources. However, by fixing the temperatures of these sources, Carnot's theoretical machine is the one that manages to have the highest efficiency.
The Carnot cycle is an idealized, reversible cycle, in which the operating fluid is a perfect gas, which corresponds to two transformations. isotherms it's two adiabatic, interspersed. The processes described by the gas in this cycle are:
Do not stop now... There's more after the advertising ;)
1.°) isothermal expansion DA, during which the gas is in contact with the constant temperature system TA (hot source), receiving from it a quantity of heat QA.
2.°) adiabatic expansion AB, during which there is no heat exchange with the environment. The system performs work with a decrease in internal energy and, therefore, in temperature.
3.°) BC isothermal contraction, during which the gas is in contact with the constant temperature system TB (cold source), giving it a quantity of heat QB.
4.°) adiabatic contraction CD, during which the gas does not exchange heat with the environment. The system receives work, which serves to increase its internal energy and therefore its temperature.
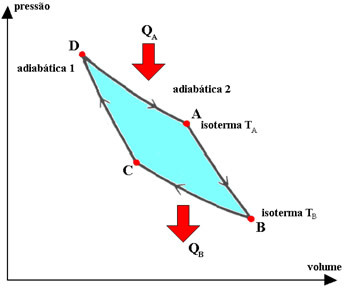
In the Carnot cycle, heat exchanged (QTHE and QB) and thermodynamic temperatures (TTHE and TB) of the hot and cold sources are proportional, the relationship being:

Substituting in the efficiency equation of a thermal machine, we obtain, for the Carnot machine:
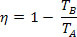
Considering the temperature of the cold source (TB) equal to zero kelvin (absolute zero), we have η = 1 or η = 100%. However, this fact contradicts the second law of thermodynamics, which guarantees that an income of 100%, which leads us to conclude that no physical system can have a temperature equal to zero absolute.
By Domitiano Marques
Graduated in Physics
Would you like to reference this text in a school or academic work? Look:
SILVA, Domitiano Correa Marques da. "Carnot Machines"; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/maquinas-carnot.htm. Accessed on June 27, 2021.