The main radioactive emissions are alpha (α), beta (β) and gamma (γ). In this article, we will talk about the first of these three radiations, how it was discovered, what it consists of, how its radiation affects the structure of matter, what is its penetration power and what damage it causes to the being human.
- Discovery:
In 1900, independently and at almost the same time, New Zealand physicist Ernest Rutherford (1871-1937) and the French chemist Pierre Curie (1859-1906) were able to experimentally identify alpha and beta.
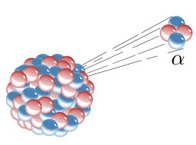
Rutherford performed a famous experiment in which he set up an apparatus similar to the one shown in the illustration below:
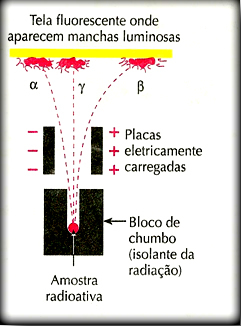
He placed a sample of a radioactive element in a lead block with an orifice. Since lead blocks radioactive emissions, they would not spread through the environment, but would be directed to exit towards the only opening in the lead. This device was placed inside a container subjected to vacuum. Two plates electrified with opposite charges were fitted to this device – that is, an electrical potential was applied. On the wall opposite the lead block, a photographic plate or a screen with zinc sulfide, a fluorescent material, which would record the radioactive emissions, was placed.
One of the factors observed with this experiment was that the path of alpha radiation was diverted to the negative pole of the plate. As is well known, opposite charges attract, consequently, it was concluded that alpha radiations are actually positive particles.
- Constitution:
Over time, it was discovered that these positive particles are actuallyformed by two protons and two neutrons (42α2+), that is, equal to a helium nucleus (42He). In addition, they are heavy particles, with high mass, as they were deflected by the electromagnetic field.
Do not stop now... There's more after the advertising ;)
- Consequences of alpha particle emission for the structure of the atom:
As we know, the emission of radiation is a process that takes place from the nucleus – hence the term nuclear reactions. Therefore, it involves a change in nuclear charge (positive), causing changes in the substance.
In the case of emission of an alpha particle (42α2+), the atomic number (number of protons) of the atom decreases two units (because it lost two protons) and its mass number (number of protons and neutrons in the nucleus) decreases four units.
See how this occurs in the emission of an alpha particle from an atom of a generic element (ZTHEX):
ZTHEX → 42α2+ + Z-2A-4X
Example:
92238U → 42α2+ + 90234Th

Alpha radiation also has a high ionizing power, being able to capture two electrons and become a helium atom:
42α2+ + 2 and- → 42he
- Penetration power:
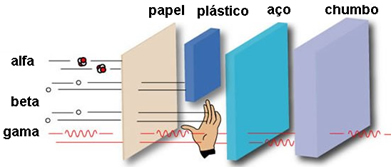
The velocity of alpha particles is low, initially being 3000 km/s up to 30 000 km/s. Its average speed is approximately 20 000 km/s, which is 5% of the speed of light. Because alpha radiation is slow, it has a very low penetration power, not penetrating even a sheet of paper, clothing or skin.
See the figure below for a comparison of its penetration power with other beta and gamma emissions:
- Damage to humans:
Due to their low penetration power, the damage that alpha particles cause to humans is small. When they affect our body, they are held back by the layer of dead skin cells, and they can, at most, cause burns.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Alpha (α) emission"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/emissao-alfa.htm. Accessed on June 27, 2021.