Substitution (syntheses) reactions are those that occur when a group of a given reagent changes position with a group of another reagent. They happen most commonly in high stability compounds (those with saturated chains). Often these syntheses occur with the involvement of heat or ultraviolet light in the reaction medium.
You alkanes they are organic compounds that are prone to undergo substitution reactions, as they have only saturated chains (only simple bonds between carbon atoms). They are widely used for syntheses that originate organic halides, such as methyl chloride (gas used as an anesthetic).
The most common substitution reactions with alkanes are:
halogenation;
nitration;
sulphonation;
a) Halogenation
It is a synthesis in which the alkane molecule reacts with a halogen molecule (Cl2, br2, I2 and F2). The use of I2 it is not so viable because it promotes an extremely slow reaction. The use of F2 it is not recommended because it is an explosive reaction that destroys organic matter.
For the halogenation of alkanes with Br
2 or Cl2 happen, the presence of light is required (λ) or strong heating. Regardless of the halogen used, the end product of this substitution reaction will always be a organic halide. The substitution will then take place between a hydrogen atom of an alkane carbon and a halogen atom, resulting in an organic halide and an acid halide (inorganic acid). See an example:
If the alkane has a number of carbons greater than two, we will have the replacement of a hydrogen by a halogen according to the following order of priority:
H in tertiary carbon > H in secondary carbon > H in primary carbon
In the following example, we can see that the H of the secondary carbon has been replaced by an atom of Br.
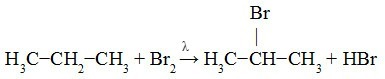
Observation: whenever the alkane has more than two carbons, more than one organic halide will be formed. The amount of halides formed will follow the order of priority. See some examples:
Butane Bromination:
We observe in the equation below the formation of 2-bromo-butane (in greater quantity as it is a priority in the substitution due to the exchange being carried out on the secondary carbon) and 1-bromo-butane (to a lesser extent the amount).
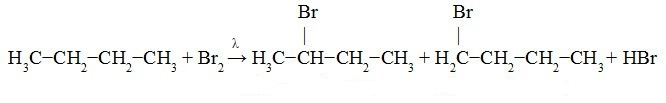
2-bromo-butane 1-bromo-butane
Chlorination of methyl propane:

2-chloro-1-chloro-
2-methyl-2-methyl-
propane propane
Do not stop now... There's more after the advertising ;)
b) Nitration
In this reaction, nitric acid (HNO3) reacts with alkane by exchanging an alkane hydrogen for a nitro group (NO2) of the acid, which results in a nitro compound and in a water molecule. As we have the presence of an acid, it is not necessary to use a catalyst.
Observation: the same priority rules used for hydrogen exchange in halogenation are used in nitration.
Track pentane nitration:
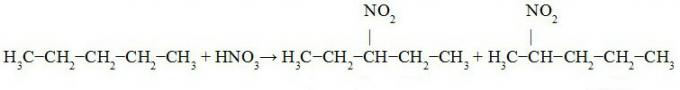
3-nitro-pentane 2-nitro-pentane

1-nitro-pentane
We observed in the equation above the formation of 3-nitro-pentane (in greater quantity as it is a priority in the replacement due to the exchange be performed on tertiary carbon), 2-nitro-pentane (exchange of hydrogen on secondary carbon) and 1-nitro-pentane (in minor the amount).
c) Sulphonation
In this reaction, sulfuric acid (H2ONLY4) reacts with alkane by exchanging an alkane hydrogen for a sulfonic group (SO3H) of acid, which results in a sulfonic acid and a water molecule. As we have the presence of an acid, it is not necessary to use a catalyst.
Observation: the same priority rules used for hydrogen exchange in halogenation are also used in sulfonation.
Track pentane sulphonation:

Penta-3-sulfonic acid Penta-2-sulfonic acid
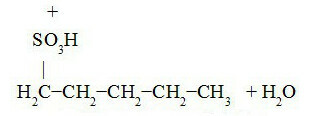
Penta-1-sulfonic acid
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Replacement reactions in alkanes"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/reacoes-substituicao-alcanos.htm. Accessed on June 28, 2021.
Chemistry

Alkanes, hydrocarbons, methane, saturated chain, aliphatic, paraffins, fuels, gasoline, mineral wax, petroleum, oil shale, natural gas, petrochemical industry.
Alkanes nomenclature, hydrocarbon function, carbon valences, International Union of Pure and Applied Chemistry, IUPAC, saturated aliphatic hydrocarbons, single bonds, compounds Organic.
Chemistry
Click here and learn more about substitution reaction, a chemical process in which the reagents (organic and inorganic) used exchange one of its components with each other, forming new substances. Among the substances most used as reagents are alkanes, benzene and organic halides.


