the atom is the basic unit of matter, that is, the smallest portion into which an element can be divided without losing its chemical properties.
Atoms are formed by a nucleus composed of particles of protons and neutrons and electrons that orbit the nucleus, forming the electrosphere.
The word atom is of Greek origin and means "indivisible". Until the nineteenth century, it was believed that the atom was the smallest part of matter, that is, that it would be impossible to divide it.
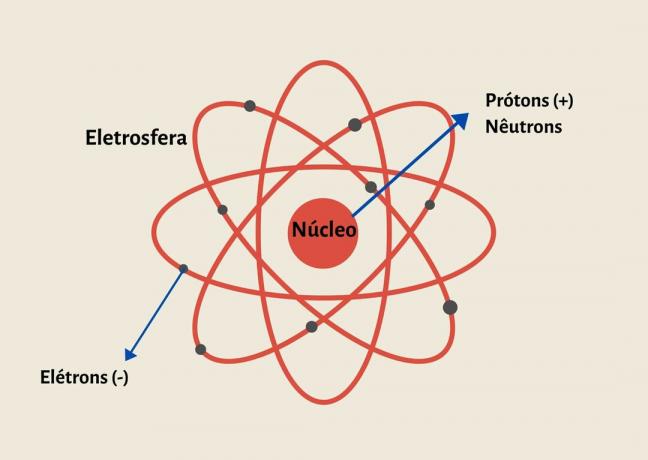
An atom is made up of protons and neutrons in the nucleus and electrons in the electrosphere.
Structure and composition of an atom
Atoms are very small pieces of matter, so small that they cannot be seen with ordinary microscopes.
Its structure is formed by an infinitely small and dense nucleus, composed of protons and neutrons, and an electrosphere composed of electrons.
- Protons (p): positive particles and with unit mass.
- Neutrons (n): neutral particles (uncharged) and with unit mass.
- Electrons (e): Negative and practically massless particles in constant orbital motion around the nucleus.
The nucleus represents 99.9% of the mass of an atom. The mass of electrons is practically irrelevant: an electron has a mass 1836 times less than the mass of protons and neutrons.
The movement of electrons around the nucleus forms a electromagnetic field. Electrons orbit around the nucleus at such a high velocity that, if you could see the atom, the electrosphere would be seen as a cloud around the nucleus.
Atoms are electrically neutral - they have the same absolute value as protons (+) and electrons (-), so their charge becomes zero.
If an atom receives or loses electrons, it ceases to be an atom and becomes a ion, which can have a positive or negative charge:
- If it receives electrons, it becomes negatively charged and becomes a anion.
- If it loses electrons, it becomes positively charged and becomes a cation.
understand what it is matter and learn more about the cations and anions.
Electrosphere structure
The electrosphere is formed by electrons in orbital motion, but these electrons are not randomly arranged, there are electronic layers where these particles are distributed.
An atom can have up to seven electronic layers. Each of these layers has different energy levels, with the outermost layer being the most energetic layer.
These layers are represented by the following letters: K, L, M, N, O, P, Q. K being the layer closest to the core.
Not all atoms have 7 layers, mercury, for example, has only 6. But regardless of the number of shells, it is a rule that the last one cannot have more than 8 electrons.
The electronic layers are further divided into energy sublevels, represented by the letters: s, p, d, f.
Atom history and atomic models
The idea that matter could be divided into small parts until it came to a unit so small that it could no longer be divided had existed since ancient Greek times.
Democritus, around 400 BC C., he was the first scientist to postulate the existence of this small particle and named it “atom”, which in Greek means “indivisible”.
It was in 1803 that the first consistent theory of atoms was developed. John Dalton argued that the atom was the smallest part of matter and that it was indivisible.
Over the following centuries, with the development of science and technology, new discoveries about this particle were made and postulated in different atomic models.
1803 - Dalton Model
Developed by Professor John Dalton in 1803, this model became known as the model of the "pool ball", because according to him the atoms were massive, indivisible and indestructible spheres.

1898 - Thomson Model
Joseph Thomson discovered the existence of electrons and, according to his model, these charges would be evenly distributed throughout the atom, with positive charges.
The atom in Thomson's model was spherical rather than massive and became known as "raisin pudding", where the raisins in a pudding represented the positive and negative charges.

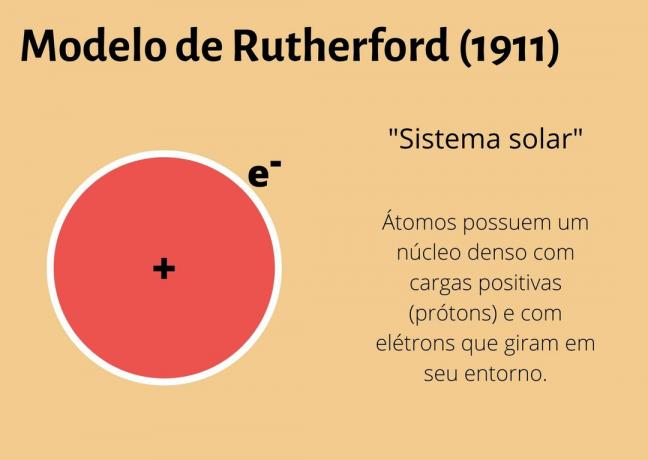
1911 - Rutherford Model
Rutherford made an important discovery about the atom: the existence of a nucleus. His model said that the atom was formed by a nucleus and an electrosphere.
In the nucleus would be the protons and neutrons and in the electrosphere the electrons. This model became known as "solar system".
What Rutherford could not explain was how electrons did not collapse with the nucleus of the atom.
1913 - Rutherford-Bohr Model
Rutherford's model was complemented with discoveries made by physicist Niels Bohr in 1913. Bohr came to the conclusion that electrons orbit the electrosphere in layers of different energy levels.
The electrons do not absorb or release energy in this movement, so they stay in a constant energy orbit, which prevents them from colliding with the nucleus.

Characteristics of an Atom
What differentiates one atom from another is the amount of protons, neutrons and electrons in its composition. The main values used to identify atoms are atomic mass and atomic number.
atomic mass
The atomic mass value is represented by the sum of the protons and neutrons present in the nucleus of the atom.
A = z + n
atomic number
The atomic number is the number of protons in the nucleus of an atom, its value is represented by the letter z. As in an atom the number of protons is equal to the number of electrons, we have:
z = p = e
The set of several atoms with the same atomic number forms a chemical element. All known chemical elements are represented on the periodic table following an increasing order of atomic number.
The chemical elements are represented in the periodic table by their acronym and name in the center, by the atomic mass at the bottom and by the atomic number at the top, as shown in the image:

- Atomic mass = 196.967
- Atomic number = 79
Atoms and molecules
An atom is a very small portion of matter, it is composed of a nucleus with protons and neutrons and electrons rotating around the nucleus.
A molecule is a grouping of atoms of the same or different elements, which together form a substance. For example:
- Two oxygen atoms unite and form an oxygen molecule (O2).
- Two hydrogen atoms unite with one oxygen atom and form a water molecule (H2O).
See too:
- Molecules
- Linus Pauling diagram