Sometimes it can happen to minimum formula be the same as the molecular formula of the compound; however, this is not always true.
For example, the empirical or minimal formula for water is H2O, indicating that there is a 2:1 ratio between the elements that make up water molecules. And, coincidentally, this is also the molecular formula for water. However, to see that this does not always happen, look at the two examples below:
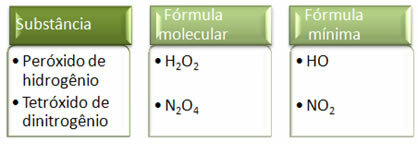
Since the minimum formula is only the ratio of the atoms of each element and not the actual amount of them in the molecular formula, it can occur of different compounds having the same empirical formula and even the minimum formula of a compound can be the same as the molecular formula of other. Note, in the example below, how this can happen:
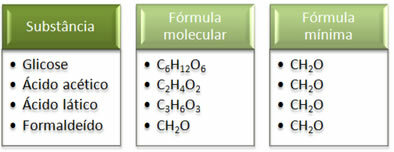
See that the minimum formula CH2The is the same for all substances, that is, this minimum formula expresses that, in all cases, the carbon, hydrogen and oxygen atoms are present in molecular formulas in a ratio of 1:2:1. Furthermore, the only one that has the same molecular formula as the empirical formula is formaldehyde.
- Calculation of the Minimum or Empirical Formula:
To determine the empirical formula of a compound, it is first necessary to know what its percentage or proximate formula is. This can be done by measuring the mass of each element in 100g of a sample. The text "Percentage or Centesimal Formula” clarifies this matter better.
Do not stop now... There's more after the advertising ;)
For example, let's say the proximate composition of a given compound is given by: 40.00% C, 6.67% H, and 53.33% O. We pass these values to grams, considering a mass of 100 g of compost sample. Thus, we have: 40 g of C, 6.67 g of H and 53.33 g of O.
Now it is necessary to pass these values to the amount of matter (mol). We do this by dividing each of the values found by their respective molar masses:
C: 40/12 = 3.33
H: 6.67/1 = 6.67
O: 53.33/16 = 3.33
Since the values are not integers, we use the following trick: we divide all values by the smallest of them, so that the proportion between them is not changed.
In this case, the smallest value is 3.33, so the result will be:
C: 3.33/3.33 = 1
H: 6.67/3.33 = 2
O: 3.33/3.33 = 1
Thus, the minimum formula of this unknown substance is equal to: Ç1H2O1 or CH2O.
Briefly, the steps needed to find the empirical or minimal formula of a substance are:

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Minimum or Empirical Formula"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/formula-minima-ou-empirica.htm. Accessed on June 28, 2021.