The polarity of a bond and a molecule is related to the distribution of electrons around atoms.If this distribution is symmetrical, the molecule will be nonpolar, but if it is asymmetrical, and one of the parts of the molecule has higher electron density, so it is a polar molecule.
The polarity of molecules can be visualized when their constituent substance is subjected to an external electric field. If molecules orient themselves in the presence of this field, that is, if one part is attracted to the positive pole and the other part of the molecule is attracted to the negative pole, then, they are polar. Otherwise, if they don't orient themselves, they are nonpolar.
For example, when you rub a glass stick with a flannel a lot, it becomes positively charged. If we approach it to a stream of water falling from a faucet, we will see that the water will not continue to fall in a straight vertical trajectory, but will be attracted by the stick, suffering a deviation. This shows that water is polar. But if we do this same experiment with a fillet of oil, it will not deviate in its trajectory, showing that its molecules are nonpolar.
By analyzing the structures of molecules, we can determine whether they are polar or not, taking into account two important factors: the difference in electronegativity between atoms and the geometry of the molecule.
1st) Electronegativity between atoms:
If the molecule is formed by bonds between atoms of the same chemical elements, that is, if they are simple substances such as O2, H2, no2, Cℓ2, P4, S8, etc., they will be non-polar, because there is no difference in electronegativity between their atoms.
The only exception is the ozone molecule (O3), which will be seen later.
If the molecule is diatomic and formed by elements of different electronegativities, then the molecule will be polar. Examples: HCℓ, HF, HBr and HI.
2nd) Molecule geometry:
The molecule's geometry affects how the electrons will be distributed in it and, consequently, its polarity. If the molecule is made up of three atoms or more, we will have to analyze each bond that is made and the geometry of the molecule. See an example: CO2 – linear molecule:
δ- δ+ δ-
O = C = O
Note that oxygen is more electronegative than carbon, so the bond electrons are more attracted to oxygen. In them a partial negative charge is formed (δ-), while in carbon a partial positive charge is formed (δ+). The multiplication of the distance between the nuclei of the atoms bonded with these charges in modulus (that is, only the number without a plus or minus sign) is called dipole moment and is represented by μ.
μ = d. |δ|
This dipole moment is indicated by arrows pointing in the direction of the most electronegative element, which attracts electrons: O ← C → O. This shows that this quantity is a vector (a quantity that has a magnitude or intensity, direction and direction). Therefore, it is best represented by:  .
.

Adding all the vectors together, we find the resulting dipole moment, 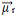 , which in this case was equal to zero because the two dipole moments have equal values, but go in opposite directions, canceling each other out.
, which in this case was equal to zero because the two dipole moments have equal values, but go in opposite directions, canceling each other out.
When the resulting dipole moment vector is equal to zero, the molecule is nonpolar, but if it is nonzero, it will be polar.
Therefore, in the case of the CO molecule2, she is apolar.
Now look at another example: H2O - angular geometry (because oxygen has two pairs of electrons available at the outermost level, which repel the electrons from bonds with hydrogens):
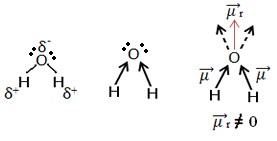
Electrons are attracted to oxygen. But, in this case, the vectors do not cancel each other out, because the molecular geometry of water is angular, since its directions are not opposite, giving a nonzero resulting dipole moment vector, and therefore the water molecule is polar.
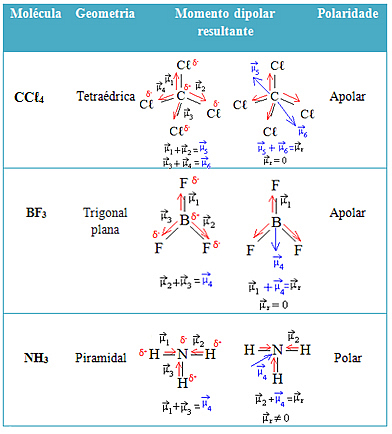
See more examples in the table below:
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/polaridade-das-moleculas.htm