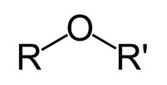
Ethers are a functional group of organic compounds that have in their structure an atom of the element oxygen between carbons.
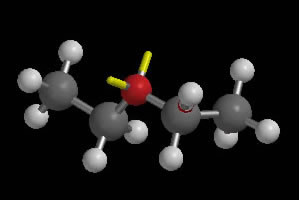
Among the main ethers, what stands out is the one commonly called “ether” or “common ether” sold in pharmacies, which, in fact, has as its official nomenclature the name “ethoxyethane”. By the usual nomenclature, its name is "ethyl ether" or "diethyl ether", whose structural formula is as follows: H3C CH2 O CH2 CH3.
This compound is also sometimes called sulfuric ether, due to its first obtainment, which was in 1540, when the German botanist Valerius Cordus carried out a reaction between ethyl alcohol and sulfuric acid.
From 1842 it began to be used as an inhalation anesthetic and for a long time it was used for this purpose in surgeries, since its vapors make people relax their muscles and cause a slight affect on blood pressure, pulse rate and breathing. It was also used as an anesthetic for tooth extraction and in major surgeries, such as the one done in the mid-19th century by John Collins, in which he removed a tumor from a patient.
Do not stop now... There's more after the advertising ;)
However, after anesthesia, ethoxyethane causes malaise, irritation in the respiratory tract, in addition to putting the surgical site at risk, possibly causing fires, as it forms a highly explosive mixture with oxygen, where the product is an organic peroxide that likely acts to detonate the explosion. Therefore, over time it was replaced by other anesthetics. Today it is still used as an anesthetic, being applied to the skin, which reduces its sensitivity, making it possible, then, to apply an injection, for example.
Currently, it is mostly used as an inert apolar solvent in organic reactions, mainly in the extraction of essences, perfumes, oils, fats, among others. In fact, it is the best solvent for extracting cocaine from coca leaves. Because of this fact, its sale is monitored by the Federal Police.
Even today, the industrial production of ethyl ether is made through ethyl alcohol and sulfuric acid, through the reaction outlined below:

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Ethoxyethane: the main ether"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/etoxietano-principal-eter.htm. Accessed on June 28, 2021.