Sublimation is the change from the solid state to the gaseous state and vice versa, without going through the liquid state.
For a substance to undergo the sublimation process, it must be subjected to certain values of temperature and pressure.
Mothballs and CO2 solid (dry-ice) are examples of substances that undergo sublimation under ambient conditions.

Phases diagram
We can discover the physical state of a substance by knowing the values of temperature and pressure to which it is subjected.
For this, we use diagrams built for each substance, from values found experimentally.
Called "Phases diagram", it is divided into three regions that represent the solid, liquid and gaseous states. The lines that delimit these regions signal the points at which the substance changes phase.
The triple point in the diagram indicates the temperature and pressure at which the substance can coexist in the three phases. Below this point is the sublimation curve.
The points on this curve determine the pressure and temperature values at which sublimation will occur.
When a solid is subjected to a pressure less than the triple point, if it is heated it will go directly into a gaseous state.
The change from the direct solid state to the gaseous state can also happen by decreasing the pressure when its temperature is lower than that of the triple point.
Learn more at: Physical State Changes.
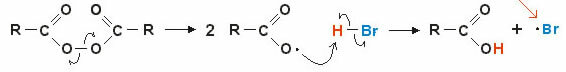
Carbon Dioxide (CO) Phase Diagram2 )
The CO triple point2 occurs when the pressure is 5 atm. This fact justifies that it is common to see the occurrence of sublimation in dry ice, since the ambient pressure is 1 atmosphere.
For this reason, liquid carbon dioxide is not obtained under ambient conditions. Under these conditions, it is either in a solid state or in a vapor state.
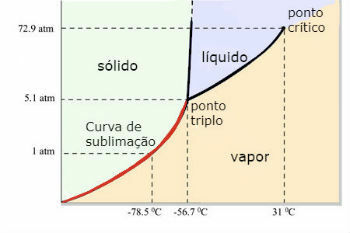
Water phase diagram (H2O)
The triple point of water occurs when the pressure is only 0.06 atm. Thus, under ambient conditions it is not common for water to sublimate.
To learn more, read also:
- Physical States of Water
- Physical States of Matter
- Liquefaction or Condensation
- Matter Properties
- Solidification
- Fusion
- Vaporization
- Boiling
- Evaporation