THE centrifuge, a siphoning and the fractional distillation are subsequent methods to the traditional ones used in the separation of mixtures.
Centrifugation
Centrifuging is nothing more than accelerating the decanting process. If the objective is to quickly separate the solid from a liquid, we can submit the mixture to a centrifuge. It spins at high speed and deposits solid particles, which are denser, at the bottom.
A practical example of this method is blood centrifugation (see main image), performed in clinical analysis laboratories. The blood is placed in compartments and then subjected to the action of a centripetal force (a force that pulls the mixture towards the center of the trajectory in a circular motion). In this way, plasma is separated from other blood components for further analysis.
Another example applies in our homes through washing machines. Have you ever noticed how your laundry is spun?
Washers have a spin device, which is activated when you want to dry your clothes. This garment rotates at high speed against the surface inside the machine. Such movement causes water to be extracted from the clothes.
Siphoning
After carrying out the decantation process shown in Dissolution, filtration and decantation, if it is not possible to pour the liquid obtained in the decanting, we can remove it with the aid of a siphon. A siphon is a specially shaped pipe, usually made of plastic or glass, used to drain water from one container to another.
Do not stop now... There's more after the advertising ;)
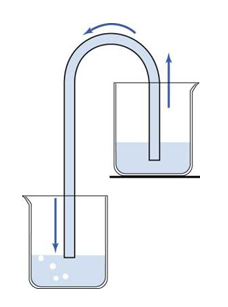
In this case, the siphon was used to transport a liquid from one height to another. In this case, the lowest, passing through a higher point. The device called “siphon” gave rise to the term siphoning.
Fractional Distillation
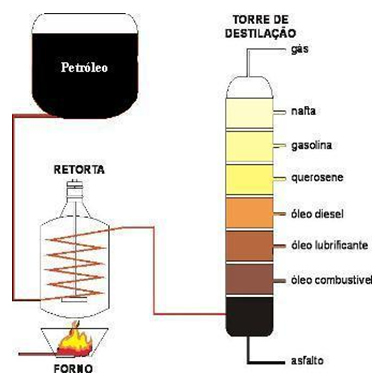
This method is much more complex than the one used in simple distillation. This process consists of heating a mixture of two or more liquids that have boiling points not very close. The solution is heated and, initially, the liquid with a lower boiling point separates. The solution continues under heating, and the liquid with a boiling point above the first is separated. And so it happens, successively, until the separation of the liquid with the highest boiling point.
Fractional distillation is the method used in petrochemical industries to separate the various derivatives of Petroleum, as shown in the following image:
By Líria Alves
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Centrifuging, siphoning and fractional distillation"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/centrifugacao-sifonagem-destilacao.htm. Accessed on June 28, 2021.