In the text "Relationship between polarity and solubility of substances", you saw that generally solutes polar substances dissolve in solvents that are also polar and that non-polar substances dissolve in solvents too non-polar. However, this is not a rule that can apply to all solubility cases.
For example, sugar dissolves in water, but oil does not. It is true that water and sugar molecules are polar, while oil molecules are non-polar, but they are the types of intermolecular forces between the molecules of these isolated substances and each other that provide us with the explanation for this fact.

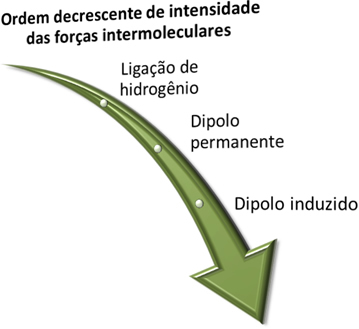
Before we see what these forces are, remember that, as a matter of intensity, stronger is the hydrogen bond, which is followed by the permanent dipole force and the weakest is the induced dipole force.
Both water and sugar molecules (sucrose - C12H22O11), present oxygen atoms bonded to hydrogen atoms, forming groups ─ O ─ H. This means that between water molecules and between sugar molecules there may be intermolecular hydrogen bond interactions.
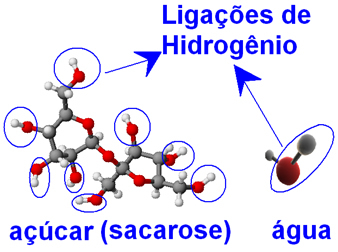
That's why the water molecules are able to wrap the sugar molecules that were tightly bound together in the form of crystals and separate them, preventing them from rejoining. Thus, sugar has great solubility in water, and we can dissolve up to 33 g of it in 100 g of water at 20ºC.
Now oil and water are immiscible. This does not mean that the oil is not attracted to the water, as the fact that it spreads over the surface of the water rather than being in the shape spherical, reveals to us that it is looking for a shape in which a greater amount of oil molecules are in contact with the oil molecules. Water.
Do not stop now... There's more after the advertising ;)
However, the attraction between water molecules is much greater (hydrogen bonding) than the attraction between oil and water molecules. Therefore, oil molecules cannot break the bond between two neighboring water molecules.
This leads us to conclude that:
“If the existing intermolecular force is more intense than the possible new interaction, then the solute does not solubilize, the original bond remaining. But if the new interaction is stronger, the solute will solubilize, breaking the substances' intermolecular bonds.”
Another example that shows us the importance of intermolecular forces for the solubility of materials is when we have iodine, water and benzene. In the diagram below, we have that iodine dissolves well in benzene and is slightly soluble in water, water and benzene are totally immiscible and when we have a mixture of benzene and water and then we add the iodine, it only dissolves in the benzene:
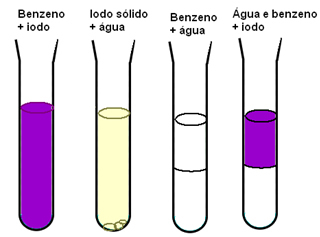
Benzene and iodine are non-polar, having an easier time mixing than water, which is polar. But what really explains what happens is that the intermolecular induced dipole forces existing between the nonpolar molecules are weak compared to the hydrogen bonds of water.
Therefore, since existing interactions between water molecules are stronger than possible new ones interactions, the hydrogen bonds are not broken and a two-phase system is observed when mixing benzene and Water.
The new interactions that are formed between the iodine molecules and the benzene molecules are more intense than those that occur between the molecules of these isolated substances.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Relationship between Intermolecular Strength and Solubility of Substances"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/relacao-entre-forca-intermolecular-solubilidade-das-substancias.htm. Accessed on June 27, 2021.


