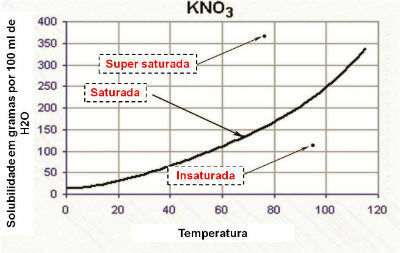
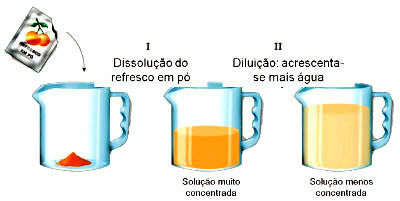
Also called mineral chemistry, it is the branch of chemistry that studies the chemical elements and substances in nature that do not have carbon coordinated in chains.
Comprising 95% of the substances on the planet, inorganic substances are divided into four groups:
• Acids that are substances that, when coming into contact with a certain aqueous solution, release H+ ions.
• Bases or Hydroxides that release OH- ions to a given solution.
• Salts that are formed by the reaction between an acid and a base. It is composed of a cation other than H+ bonded to an anion other than OH-.
• Oxides which is a binary compound formed by oxygen atoms with other elements, the most electronegative element being oxygen.
The first electric cell appeared in 1800 by the Italian scientist Volta. After its discovery, a period of experiments began, and among them one consisted of dipping the tips of two conductor wires connected to a battery in different solutions interspersing a light bulb in the circuit. proof.
They then observed that some solutions carried electrical current and others did not. An aqueous solution of table salt was found to conduct an electric current. Several theories tried to explain this fact, but only Arrhenius' one was accepted.
Arrhenius Theory
The theory says that a substance dissolved in water breaks down into smaller and smaller particles, but in in some cases the division into molecules breaks down and then the solution cannot carry current electric.
On the other hand, division can go beyond molecules by dividing into micro particles called ions carrying an electrical current.
Do not stop now... There's more after the advertising ;)
Inorganic chemistry - Chemistry - Brazil School
Would you like to reference this text in a school or academic work? Look:
SCHOOL, Team Brazil. "Definition of Inorganic Chemistry"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/definicao-quimica-inorganica.htm. Accessed on June 28, 2021.